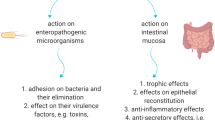
Abstract
Saccharomyces boulardii (S. boulardii) is a non-pathogenic biotherapeutic agent, widely prescribed in a lyophilized form in many countries over the world. S. boulardii acts as a shuttle liberating effective enzymes, proteins and trophic factors during its intestinal transit that improve host immune defenses, digestion, and absorption of nutrients. In addition, S. boulardii secretes during its intestinal transit polyamines, mainly spermine and spermidine that regulate gene expression and protein synthesis. In this review, we will focus on the interactions of the yeast with the host intestinal mucosa.





Similar content being viewed by others
References
McFarland LV, Bernasconi P (1993) Saccharomyces boulardii: A review of an innovative biotherapeutic agent. Microbiol Ecol Health Dis 6:151–171
Elmer GW, Surawicz CM, McFarland LV (1996) Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal secretions. JAMA 275:870–876
Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, Van Belle G (1989) Prevention of antibiotic associated diarrhoea by Saccharomyces boulardii: A prospective study. Gastroenterology 96:981–988
McFarland LV, Surawicz CM, Greenberg RN, et al. (1995) Prevention of ß–lactam associated diarrhoea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol 90:439–448
McFarland LV, Surawicz CM, Greenberg RN, et al. (1994) A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 271:1913–1918
Surawicz CM, McFarland LV, Greenberg R, et al. (2000) The search for a better treatment for Clostridium difficile disease: Use of high-dose Vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 31:1012–1017
Buts JP, Corthier G, Delmée M (1993) Saccharomyces boulardii for Clostridium difficile-associated enterocolopathies in infants. J Pediatr Gastroenterol Nutr 16:1497–1504
Höchter W, Chase D, Hegenhoff G (1990) Saccharomyces boulardii in treatment of acute adult diarrhoea. Efficacy and tolerance of treatment. Münch Med Wochen 132:188–192
Cetina-Sauri G, Sierra Basto G (1994) Evaluation thérapeutique de Saccharomyces boulardii chez des enfants souffrant de diarrhée aiguë. Ann Pediatr 41:397–400
Kollaritsch H, Holst H, Grobara P, et al. (1993) Prophylaxe der Reisediarrhoë mith Saccharomyces boulardii. Forst Chr Med 111:152–156
Buts JP (2004) Exemple d’un médicament probiotique Saccharomyces boulardii lyophylisé. In Rambaud JC, Buts JP, Corthier G, Fourié B (eds) Flore microbienne intestinale: Physiologie et pathologie digestives. John Libbey Eurotext, Montrouge, France, pp 221–244
Bleichner G, Bléhaut H, Mentec H, Moyse D (1997) Sacchromyces boulardii prevents diarrhoea in critically ill tube-fed patients. A multicenter, randomized, double-blind–placebo-controlled trial. Intensive Care Med 23:517–523
Saint-Marc T, Bléhaut H, Musial C, Touraine JL (1991) Efficacité de Saccharomyces boulardii dans le traitement des diarrhées du SIDA. Am Med Int 142:64–65
Guslandi M, Mezzi G, Sorghi M, Testoni PA (2000) Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci 45:1462–1464
Guslandi M, Giollo P, Testoni PA (2003) A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol 15:697–698
Hennequin C, Thierry A, Richard GF, et al. (2001) Microsatellite typing as a new tool for identification of Saccharomyces cerevisiae strains. J Clin Microbiol 39:551–559
Maillée M, Van Nguyen P, Bertout S, Vaillant C, Bastide JM (2001) Genotypic study of Saccharomyces boulardii compared to the Saccharomyces sensu stricto complex species. J Mycol Med 11:19–25
Bergogne-Berezin E (1995) Impact écologique de l’anti-biothérapie. Place des micro-organismes de substitution dans le contrôle des diarrhées et colites associées aux antibiotiques. Press Méd 24:145–156
Buts JP (1999) Mechanisms of action of biotherapeutic agents. In Elmer GW, McFarland LV, Surawicz CM (eds) Biotherapeutic agents and infectious disease. Humana Pres, Totowa, NJ, pp 27–46
Bléhaut H, Massot J, Elmer GW, Levy RM (1989) Disposition kinetics of Saccharomyces boulardii in man and rat. Biopharm Drug Dispos 10:353–364
Pothoulakis C, Kelly CP, Joshi MA, et al. (1993) Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 104:1108–1115
Castagliulo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C (1999) Saccharomyces boulardii protease mediates Clostridium difficile toxin A ad B effects in human colonic mucosa. Infect Immun 67:302–307
Czerucka D, Roux J, Rampal P (1994) Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′,5′-cyclic monophosphate induction in intestinal cells. Gastroenterology 106:65–72
Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P (2000) Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli infected T84 cells. Infect Immun 68:5998–6004
Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P (2001) Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect Imm 69:1298–1305
Czerucka D, Rampal P (2002) Experimental effects of Saccharomyces on diarrhoeal pathogens. Microbes Infect 4:733–739
Buts JP, Bernasconi P, Van Craynest MP, Maldague P, De Meyer R (1986) Response of human and rat small intestinal mucosa to oral administration of Saccharomyces boulardii. Pediatr Res 20:192–196
Jahn HU, Ullrick R, Schneider, et al. (1996) Immunology and tropical effects of Saccharomyces boulardii on the small intestine in healthy human volunteers. Digestion 57:95–104
Harms HK, Bertele-Harms RM, Bruer-Kleis D (1987) Enzyme substitution therapy with the yeast Saccharomyces cerevisiae in congenital sucrase isomaltase deficiency. N Engl J Med 316:1306–1309
Buts JP, DeKeyser N, Stilmant C, Sokal EM, Marandi S (2002) Saccharomyces boulardii enhances N-terminal peptide hydrolysis in suckling rat small intestine by endoluminal release of a zinc-binding metalloprotease. Pediatr Res 51:528–534
Buts JP, DeKeyser N, Marandi S, Hermans D, Chae YHE, Lambotte L, Chanteux H, Tulkens PM (1999) Saccharomyces boulardii upgrades cellular adaptation after proximal enterectomy in rats. Gut 45:89–96
Marandi S, De Keyser N, Hermans D, Chae YHE, Lambotte L, Buts JP (1999) Saccharomyces boulardii upgrades cellular adaptation after proximal enterectomy in rats. Gastroenterology 116:G2382
Zaouche A, Loukil C, de la Gausie P, et al. (2000) Effects of oral Saccharomyces boulardii on bacterial overgrowth, translocation and intestinal adaptation after small bowel resection in rats. Scand J Gastroenterol 35:160–165
Buts JP, Bernasconi P, Vaerman JP, Dive C (1990) Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci 35:251–256
Qamar A, Aboudola S, Warny M, Michetti P, Pothoulakis C, LaMont JT, Kelly CP (2001) Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Imm 69:2762–2765
Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53:749–790
Pegg AE, McCamm PP (1982) Polyamine metabolism and function. Am J Physiol 243:C212–C221
Pegg AE (1986) Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J 243:249–262
Buts JP (1996) Polyamines in milk. Ann Nestlé 54:18–104
Wang JY, Viar MJ, McCormack SA, Johnson LR (1992) Effect of putrescine on S-adenosylmethionine decarboxylase in a small intestinal crypt cell line. Am J Physiol 263:G494–G501
Buts JP, Theys S, De Keyser N, Dive C (1989) Changes in serum and intestinal diamine oxidase (DAO) activity after proximal enterectomy in rats. Correlation of DAO activity with mucosal mass parameters. Dig Dis Sci 34:1393–1398
Pegg AE, Hibasami H, Matsui I, Bethell DR (1980) Formation and interconversion of putrescine and spermidine in mammalian cells. Adv Enzyme Regul 19:427–451
Panagiotidis CA, Artandi S, Calame K, Silvertein SJ (1995) Polyamines after sequence specific DNA–protein interactions. Nucleic Acid Res 23:1800–1809
Hampel KJ, Crosson P, Lee JS (1991) Polyamines favor DNA triplex formation at neutral pH. Biochemistry 30:4455–4459
Person L, Holm I, Heby O (1998) Regulation of ornithine decarboxylase mRNA translation by polyamines. J Biol Chem 263:3528–3533
Kahana C, Nathans D (1985) Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem 260:15390–15393
Buts JP, De Keyser N, De Raedemaeker L (1994) Saccharomyces boulardii enhances rat intestinal enzyme expression by endoluminal release of polyamines. Pediatr Res 36:522–527
Buts JP, De Keyser N, Kolanowski J (1993) Maturation of villus and crypt cell functions in rat small intestine: Role of dietary polyamines. Dig Dis Sci 38:1091–1098
Miret JJ, Solari AJ, Barderi PA, Goldemberg SH (1992) Polyamines and cell wall organisation in Saccharomyces cerevisiae. Yeast 8:1033–1041
Buts JP, De Keyser N, De Raedemaeker L (1995) Polyamine profiles in human milk, infant, artificial formulas and semi-elemental diets. J Pediatr Gastroenterol Nutr 21:44–49
Kelly D, King TP, Brown DS, McFadyen M (1991) Polyamine profiles of porcine milk and of intestinal tissue of pigs during suckling. Reprod Nutr Dev 31:73–80
Bardocz S, Brown DS, Garant G, Pusztai A (1990) Luminal and basolateral polyamine uptake by rat small intestine stimulated to grow by phaseolus vulgaris pectin photohaemagglutinin in vivo. Biochem Biophys Acta 1034:46–52
Kumagaï J, Jain R, Johnson LR (1989) Characteristics of spermidine uptake by isolated rat enterocytes. Am J Physiol 256:G905–G910
Kobayashi M, Iseki K, Saitoh H, Miyazaki K (1992) Uptake characteristics of polyamines into rat intestinal brush border membrane. Biochem Biophys Acta 1105:177–183
Dufour C, Dandrifosse G, Forget P, et al. (1988) Spermine and spermidine induce intestinal maturation in the rat. Gastroenterology 95:112–116
Bardocz S, Duguid TJ, Brown DS (1995) The importance of dietary polyamines in cell regeneration and growth. Br J Nutr 73:819–828
Osborne DL, Seifel ER (1989) Microflora-derived polyamines modulate obstruction-induced colonic mucosal hypertrophy. Am J Physiol 256:G1049–G1057
Girard P, Pansart Y, Lorette I, Gillardin JM (2003) Dose-response relationship and mechanism of action of Saccharomyces boulardii in castor-oil-induced diarrhoea in rats. Dig Dis Sci 48:770–774
Schneider SM, Girard-Pipau F, Filippi J, Hebuterne X, Krab K, Nano JL, Piche T, Rampal P (2002) Effects of Saccharomyces boulardii on the intestinal flora of patients on long-term enteral nutrition. Gastroenterology 122:A459 (abstract)
Acknowledgments
This work was supported by the Fonds de Recherche Scientifique Médicale (Grants 9.4524.91-4.511.92) and by a grant from Biocodex Laboratories, Montrouge, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buts, JP., De Keyser, N. Effects of Saccharomyces boulardii on Intestinal Mucosa. Dig Dis Sci 51, 1485–1492 (2006). https://doi.org/10.1007/s10620-005-9016-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-9016-x