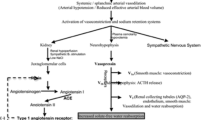
Abstract
In healthy subjects, arterial pressure reduction or renal ischemia produces renal artery dilatation through autoregulation and tubuloglomerular feedback (TuGF). Patients with decompensated cirrhosis have reduced kidney perfusion pressure but show renal vasoconstriction instead of autoregulation-mediated vasodilation. This study investigates the consequences of kidney autoregulation loss on renal perfusion, glomerular filtration rate, and tubular handling of electrolytes in both compensated and ascitic nonazotemic cirrhotic patients. Forty-two consecutive patients with diuretic-free liver cirrhosis (32 with preascitic and 10 with ascitic disease) and 10 controls were submitted to the following determinations: (a) basal plasma renin activity and aldosterone levels; (b) endogenous dopaminergic activity measured as incremental aldosterone responses during metoclopramide administration; and (c) renal clearances of sodium, potassium, inulin, para-aminohippurate and lithium. Compared with the other groups, ascitic patients showed lower renal plasma flow (P < 0.01) and lithium clearance (P < 0.05), a higher filtration fraction (P < 0.01), and secondary aldosteronism. Controls and preascitic patients displayed tubuloglomerular feedback (the mechanism increasing the glomerular filtration rate when a reduced sodium load reaches the distal tubule), as demonstrated by negative correlations between fractional excretion of lithium (an expression of fractional delivery of sodium to the distal nephron) and glomerular filtration rate (respectively, r = −0.73, P < 0.03, and r = −0.48, P < 0.01). Conversely, patients with ascites showed a positive correlation between lithium fractional excretion and glomerular filtration rate (r = 0.64, P < 0.05). Reduction in renal perfusion, increased filtration fraction, and TuGF derangement, as found in decompensated patients, are indicative of prevalent postglomerular arteriolar vasoconstriction, with ensuing stimulation of proximal tubular sodium reabsorption.
Similar content being viewed by others
References
Navar LG: Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol 234:F357–F370, 1978
Hall JE, Guyton AC, Jackson TE, Coleman TG, Lohmeier TE, Trippodo NC: Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol 233:F366–F372, 1977
Steinhausen M, Endlich K, Wiegman DL: Glomerular blood flow. Kidney Int 38:769–784, 1990
Casellas D, Moore LC: Autoregulation and tubuloglomerular feedback in juxtaglomerular arterioles. Am J Physiol 258:F660–F669, 1990
Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP: Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol 260:F486–F493, 1991
Lapointe JY, Bell PD, Cardinal J: Direct evidence for Na+:2Cl−:K+ cotransportin macula densa cells. Am J Physiol 258:F1466–F1469, 1990
Schlatter E, Salomonsson M, Persson AEG, Greger R: Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na+-2Cl−-K+ cotransport. Pfluegers Arch 414:286–290, 1989
Moore LC, Casellas D: Tubuloglomerular feedback dependence of autoregulation in rat juxtaglomerular afferent arterioles. Kidney Int 37:1402–1408, 1990
Ginès P, Fernàndez-Esparrach G, Arroyo V, Rodés J: Pathogenesis of ascites in cirrhosis. Semin Liver Dis 17:175–186, 1997
Ring-Larsen H: Renal blood flow in cirrhosis: relation to systemic and portal hemodynamics and liver function. Scand J Clin Lab Invest 37:635–642, 1977
Arroyo V, Bosch J, Mauri M, Viver J, Mas A, Rivera F, Rodés J: Renin, aldosterone and renal hemodynamics in cirrhosis with ascites. Eur J Clin Invest 9: 69–74, 1979
Rimola A, Ginès P, Arroyo V, Camps J, Perez-Ayuso RM, Quintero E, Gaya J, Rivera F, Rodés J: Urinary excretion of 6-keto-prostaglandin F1α, thromboxane B2 and prostaglandin E2 in cirrhosis with ascites. Relationship to functional renal failure (hepatorenal syndrome). J Hepatol 3:111–117, 1986
Vorobioff J, Bredfeldt J, Groszmann R: Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol 244:G52–G56, 1983
Groszmann R: Hyperdynamic circulation of liver diseases forty years later: pathophysiologyand clinical consequences. Hepatology 20:1359–1363, 1994
Colombato L, Albillos A, Groszmann R: The role of central blood volume in the development of sodium retention in portal hypertensive rats. Gastroenterology 110:193–198, 1996
Sansoè G, Biava A, Silvano S, Ferrari A, Rosina F, Smedile A, Touscoz A, Bonardi L, Rizzetto M: Renal tubular events following the passage from supine to standing position in patients with compensated liver cirrhosis: loss of tubuloglomerular feedback. Gut 51:736–741, 2002
Pugh RNH, Murray-Lion IM, Dawson JL, Pietroni MC, Williams R: Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649, 1973
Minetti EE, Cozzi MG, Biella E, Napoli F, Guidi E: Evaluation of a short protocol for the determination of para-aminohippurate and inulin clearances. J Nephrol 7:342–346, 1994
Cole BR, Giangiacomo J, Ingelfinger JR, Robson AM: Measurement of renal function without urine collection. A critical evaluation of the constant-infusion technic for determination of inulin and para-aminohippurate. N Engl J Med 287:1109–1114, 1972
Gniadek TC, Grekin RJ, Gross MD, Gross MD, Villareal JZ: Hyper-responsiveness of aldosterone to metoclopramide in aldosteronism. Clin Endocrinol 16:475–481, 1982
Ganguly A, Pratt JH, Weinberger MH, Grim CE, Fineberg NS: Differing effects of metoclopramide and adrenocorticotropin on plasma aldosterone levels in glucocorticoid-suppressible hyperaldosteronism and other forms of hyperaldosteronism. J Clin Endocrinol Metab 57:388–392, 1983
Bartels H, Bohmer M: Eine mikromethode zur kreatinibestimmung. Clin Chim Acta 32:81–85, 1971
Roe JH, Epstein JH, Goldstein NP: A photometric method for the determination of inulin in plasma and urine. J Biol Chem 178:839–845, 1949
Smith HW, Finkelstein N, Alminosa L, et al.: The renal clearance of substituted hippuric acid derivatives and other aromatic acids in dog and men. J Clin Invest 24:388–403, 1945
Thomsen K, Olsen OV: Renal lithium clearance as a measure of the delivery of water and sodium from the proximal tubule in humans. Am J Med Sci 288:158–161, 1984
Boer WH, Koomans HA, Dorhout-Mees EJ: Lithium clearance during paradoxical natriuresis of hypotonic expansion in man. Kidney Int 32:376–381, 1987
Sansoè G, Ferrari A, Baraldi E, Castellana CN, De Santis MC, Manenti F: Renal distal tubular handling of sodium in central fluid volume homoeostasis in preascitic cirrhosis. Gut 45:750–755, 1999
Kirkwood BR: Non-parametric methods. In BR Kirkwood (ed). Essentials of Medical Statistics. Cambridge, MA, Blackwell Science, 1988, pp 147–152
Siegel S: Measures of correlation and their tests of significance. In S Siegel (ed). Nonparametric statistics for the behavioral sciences. New York, McGraw–Hill, 1956, pp 195–239
Boer WH, Koomans A, Dorhout Mees EJ, Gaillard CA, Rabelink AJ: Lithium clearance during variations in sodium intake in man: effects of sodium restriction and amiloride. Eur J Clin Invest 18:279–283, 1988
Sansoè G, Ferrari A: Letter to the Editor. Renal sodium handling in preascitic cirrhosis. Gut 48:740–741, 2001
Lieberman FL, Reynolds TB: Plasma volume in cirrhosis of the liver: its relation to portal hypertension, ascites and renal failure. J Clin Invest 46:1297–1308, 1967
Wong F, Liu P, Tobe S, Morali G, Blendis L: Central blood volume in cirrhosis: measurement with radionuclide angiography. Hepatology 19:312–321, 1994
Lewis FW, Adair O, Rector WG Jr: Arterial vasodilatation is not the cause of increased cardiac output in cirrhosis. Gastroenterology 102:1024–1029, 1992
Wilkinson SP, Smith IK, Clarke M, Arroyo V, Richardson J, Moodie H, Williams R: Intrarenal distribution of plasma flow in cirrhosis as measured by transit renography: relationship with plasma renin activity and sodium and water retention. Clin Sci Mol Med 52:469–475, 1977
Wilkinson SP, Smith IK, Williams R: Changes in plasma renin activity in cirrhosis: a reappraisal based on studies in 67 patients and “low renin” cirrhosis. Hypertension 1:125–129, 1979
Trevisani F, Bernardi M, Gasbarrini A, Tamè MR, Giancane S, Andreone P, Baraldini M, Cursaro C, Ligabue A, Gasbarrini G: Bed-rest-induced hypernatriuresis in cirrhotic patients without ascites: does it contribute to maintain “compensation”? J Hepatol 16:190–196, 1992
Rector WG Jr, Adair O, Hossack KF, Rainguet S: Atrial volume in cirrhosis: relationship to blood volume and plasma concentration of atrial natriuretic factor. Gastroenterology 99:766–770, 1990
Krishna GC, Danovitch GM, Sowers JR: Catecholamine responses to central volume expansion produced by head-out water immersion and saline infusion. J Clin Endocrinol Metab 56:998–1002, 1983
Krishna GC, Danovitch GM, Beck FWJ, Sowers JR: Dopaminergic mediation of the natriuretic response to volume expansion. J Lab Clin Med 105:214–218, 1985
Sansoè G, Ferrari A, Baraldi E, Grisolia C, De Santis MC, Villa E, Manenti F: Endogenous dopaminergic activity in Child-Pugh A cirrhosis: potential role in renal sodium handling and in the maintenance of clinical compensation. Eur J Clin Invest 28:131–137, 1998
Wong F, Liu P, Blendis L: The mechanism of improved sodium homeostasis of low-dose losartan in preascitic cirrhosis. Hepatology 35:1449–1458, 2002
Wernze H, Spech HJ, Muller G: Studies on the activity of renin-angiotensin-aldosterone system (RAAS) in patients with cirrhosis of the liver. Klin Wochenshr 56:390–397, 1978
Bernardi M, Trevisani F, Santini C, De Palma R, Gasbarrini G: Aldosterone related blood volume expansion in cirrhosis before and during the early phase of ascites formation. Gut 8:761–766, 1983
Bernardi M, Trevisani F, Gasbarrini G: The renin-angiotensin-aldosterone system in liver disease. In A Bomzon, LM Blendis (eds). Cardiovascular Complications of Liver Disease. Boca Raton, FA, CRC Press, 1990, pp 29–62
Trevisani F, Colantoni A, Sica G, Gasbarrini A, D’Intino PE, De Notariis S, De Iaso R, Barbieri A, Morselli A, Gasbarrini G: High plasma levels of atrial natriuretic peptide in preascitic cirrhosis: indirect evidence of reduced natriuretic effectiveness of the peptide. Hepatology 22:132–137, 1995
Bernardi M, Di Marco C, Trevisani F, De Collibus C, Fornalè L, Baraldini M, Andreone P, Cursaro C, Zacà F, Ligabue A, Gasbarrini G: The hemodynamic status of preascitic cirrhosis: an evaluation under steady-state condition and after postural change. Hepatology 15:341–346, 1992
Salò J, Ginès A, Anibarro L, Jimenez W, Bataller R, Clària J, Ginès P, Rivera F, Arroyo V, Rodés J: Effect of upright posture and physical exercise on endogenous neurohumoral systems in cirrhotic in cirrhotic patients with sodium retention and normal supine plasma renin, aldosterone, and norepinephrine levels. Hepatology 22:479–487, 1995
Gerbes AL, Wernze H, Arendt RM, Riedel A, Sauerbruch T, Paumgartner G: Atrial natriuretic factor and renin-angiotensin-aldosterone in volume regulation of patients with cirrhosis. Hepatology 9:417–422, 1989
Epstein M, Levinson R, Sancho J, Haber E, Re R: Characterization of the renin-aldosterone system in decompensated cirrhosis. Circ Res 41:818–829, 1977
Shapiro MD, Nicholls KM, Groves MB, Kluge R, Chung HM, Bichet DG, Schrier RW: Interrelationship between cardiac output and vascular resistance as determinants of effective arterial blood volume in cirrhotic patients. Kidney Int 28:206–211, 1985
Bernardi M, Fornalè L, Di Marco C, Trevisani F, Baraldini M, Gasbarrini A, De Collibus C, Zacà F, Ligabue A, Colantoni A, Gasbarrini G: Hyperdynamic circulation of advanced cirrhosis: a reappraisal based on posture-induced changes in hemodynamics. J Hepatol 22:309–318, 1995
Wood LJ, Massie D, McLean AJ, Dudley FJ: Renal sodium retention in cirrhosis: tubular site and relation to hepatic disfunction. Hepatology 8:831–836, 1988
Wong F, Massie D, Hsu P, Dudley FJ: Renal response to a saline load in well-compensated alcoholic cirrhosis. Hepatology 20:873–881, 1994
Wong F, Massie D, Colman J, Dudley FJ: Glomerular hyperfiltration in patients with well-compensated alcoholic cirrhosis. Gastroenterology 104:884–889, 1993
Ginès P, Rodés J: Clinical disorders of renal function in cirrhosis with ascites. In V Arroyo, P Ginès, J Rodés, RW Schrier (eds). Ascites and renal disfunction in liver disease. Cambridge, MA, Blackwell Sciences, 1999, pp 36–62
Wright FS, Mandin H, Persson AE: Studies of the sensing mechanism in the tubuloglomerular feedback pathway. Kidney Int Suppl 12:S90–S96, 1982
Blantz RC, Pelayo JC: A functional role for the tubuloglomerular feedback mechanism. Kidney Int 25:739–746, 1984
Briggs JP, Schnermann J: The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 49:251–273, 1987
Rose BD, Post TW: Clinical Physiology of Acid-Base and Electrolyte Disorders. 5th ed. New York, McGraw–Hill, 2001, pp 21–70
Arendshorst W, Gottschalk CW: Glomerular ultrafiltration dynamics: Historical perspective. Am J Physiol 248:F163–F170, 1985
Haberle DA, Ruhland G, Lausser A, Moore L, Neiss A: Influence of glomerular filtration rate on renal PAH secretion in the rat kidney. Dependency of PAH extraction on renal filtration fraction. Pflugers Arch 375:131–139, 1978
Depner TA: Suppression of tubular anion transport by an inhibitor of serum protein binding in uremia. Kidney Int 20:511–518, 1981
Ichikawa I, Pfeffer JM, Pfeffer MA, Hostetter TH, Brenner BM: Role of angiotensin II in the altered renal function of congestive heart failure. Circ Res 55:669–675, 1984
Textor SE, Novick A, Tarazi RC, Klimas V, Vidt DG, Pohl M: Critical perfusion pressure for renal function in patients with bilateral atherosclerotic renal vascular disease. Ann Intern Med 102:308–314, 1985
Ichikawa I, Harris RC: Angiotensin actions in the kidney: renewed insight into the old hormone. Kidney Int 40:583–590, 1991
Arroyo V, Bernardi M, Epstein M, Henriksen JH, Schrier RW, Rodés J: Pathophysiology of ascites and functional renal failure in cirrhosis. J Hepatol 6:239–257, 1988
Myers BD, Deen WM, Brenner BM: Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and and proximal tubule fluid reabsorption in the rat. Circ Res 37:101–110, 1975
Heyeraas KJ, Aukland K: Interlobular arterial resistance: influence of renal arterial pressure and angiotensin II. Kidney Int 31:1291–1298, 1987
Tucker BJ, Mundy CA, Blantz RC: Adrenergic and angiotensin II influences on renal vascular tone in chronic sodium depletion. Am J Physiol 252:F811–F817, 1987
Gatta A, Angeli P, Caregaro L, Menon F, Sacerdoti D, Merkel C: A pathophysiological interpretation of unresponsiveness to spironolactone in a stepped-care approach to the diuretic treatment of ascites in nonazotemic cirhotic patients. Hepatology 14:231–236, 1991
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sansoè, G., Silvano, S., Mengozzi, G. et al. Loss of Tubuloglomerular Feedback in Decompensated Liver Cirrhosis: Physiopathological Implications. Dig Dis Sci 50, 955–963 (2005). https://doi.org/10.1007/s10620-005-2671-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-2671-0