Abstract
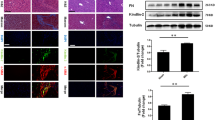
Hepatic stellate cells (HSCs), as the most important stromal cells in the liver microenvironment, play crucial roles in hepatic fibrosis, hepatocellular carcinoma, liver regeneration and fetal liver development after transdifferentiating into myofibroblasts (MFs). Transforming growth factor β1 (TGF-β1), as an important polyergic cytokine, is involved in HSCs activation process. However, the specific mechanisms of HSCs transdifferentiation process are not clearly demonstrated. Here we added exogenous recombinant TGF-β1 protein and transforming growth factor β receptor 1 (TGF-βR1) inhibitor SB431542 into mouse HSCs to detect the detailed impact of TGF-β1 signaling on HSCs activation. TGF-β1 signaling significantly increased phosphorylated (P)-Smad2/3 level and promoted Smad2/3 translocation from the cytoplasm to the nucleus, which also caused transdifferentiation of HSCs into MFs. Importantly, TGF-β1 signaling also resulted in high expression of Notch pathway markers Notch1, Jagged1, Hes1 in HSCs. In contrast, expression of those above markers in mouse HSCs were obviously decreased after hampering TGF-β1 signaling via TGF-βR1 inhibitor SB431542. To further examine the effect of Notch pathway on HSCs activation process, TGF-β1-stimulated HSCs and control HSCs were treated with or without LY450139, a specific inhibitor of Notch pathway. LY450139 evidently decreased the expression of Notch1 and MFs marker α-smooth muscle actin (α-SMA) expression in HSCs. These above results may provide a novel insight that TGF-β1 signaling controls HSCs activation process through regulating the expression of Notch pathway markers.




Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Bai S, Kopan R, Hilton WM, Ong C, Long F, Ross F et al (2008) NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 283:6509–6518
Brenner DA (2009) Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc 120:361–368
Chen E, Cen Y, Lu D, Luo W, Jiang H (2018) IL-22 inactivates hepatic stellate cells via downregulation of the TGF-β1/Notch signaling pathway. Mol Med Rep 17:5449–5453
Chunyue Y, Evason KJ, Kinji A, Stainier DYR (2013) Hepatic stellate cells in liver development, regeneration and cancer. J Clin Invest 123:1902–1910
Connolly MK, Bedrosian AS, Ashim M, Henning JR, Junaid I, Valery V et al (2010) In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol 185:2200–2208
Dana GP, Thomas DS, Seh-hoon O, Susan VS, Houda D, Bryon EP (2010) Hepatic stellate cells’ involvement in progenitor-mediated liver regeneration. Lab Invest J Tech Methods Pathol 90:1199–1208
Ekihiro S, Schwabe RF (2015) Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61:1066–1079
Gao R, Chen R, Yu C, Yuan W, Kang S, Zhang Y et al (2017) Emodin suppresses TGF-β1-induced epithelial–mesenchymal transition in alveolar epithelial cells through Notch signaling pathway. Toxicol Appl Pharmacol 318:1–7
Iimuro Y, Brenner DA (2008) Matrix metalloproteinase gene delivery for liver fibrosis. Pharm Res 25:249–258
Jiri Z, Lukas C, Noem SN, Ttinger EPB (2004) Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23:1155–1165
Kalinichenko VV, Dibyendu B, Yan Z, Gusarova GA, Wooram K, Brian S et al (2010) Foxf1 ± mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology 37:107–117
Kopan R, Ilagan MXG (2009) The canonical notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP et al (2000) Notch signaling is essential for vascular morphogenesis in mice. Genes Develop 14:1343
Li R, Dai G, Zhao M, Zhang Y, Hui L, Zhang X et al (2013) Preventative effect of Astragalus flavescens on hepatic fibrosis in rats and its mechanism of action. Exp Ther Med 6:904–908
Li HY, Ju D, Zhang DW, Li H, Kong LM, Guo Y et al (2015) Activation of TGF-Î21-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci Rep 5:16552
Liu Z, Wang J, Xing W, Peng Y, Huang Y, Fan X (2018) Role of DDAH/ADMA pathway in TGF-β1-mediated activation of hepatic stellate cells. Mol Med Rep 17:2549
Livak, Schmittgen (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods 25:402–408
Lorenzini S, Bird TL, Bellamy C, Samuel K, Aucott R, Clayton E et al (2010) Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 59:645–654
Miyazaki T, Bouscarel B, Ikegami T, Honda A, Matsuzaki Y (2009) The protective effect of taurine against hepatic damage in a model of liver disease and hepatic stellate cells. Adv Exp Med Biol 643:293–303
Nagahara T, Shiraha H, Sawahara H, Uchida D, Takeuchi Y, Iwamuro M et al (2015) Hepatic stellate cells promote upregulation of epithelial cell adhesion molecule and epithelial–mesenchymal transition in hepatic cancer cells. Oncol Rep 34:1169–1177
Nakano Y, Nakao S, Sumiyoshi H, Mikami K, Tanno Y, Sueoka M et al (2017) Identification of a novel alpha-fetoprotein-expressing cell population induced by the Jagged1/Notch2 signal in murine fibrotic liver. Hepatol Commun 1:215–229
Razao I, Xiaoying Z, Nathan T, Harry MS, Stephen K, Christopher B et al (2003) Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. Faseb J Off Publ Federat Am Soc Exp Biol 17:47
Roong Z, Duncan SA (2010) Embryonic development of the liver. Hepatology 41:956–967
Scott L (2008) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88:125–172
Scott L (2011) Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol 25:195–206
Shi Y, Massagué J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700
Wang W, Feng Y, Aimaiti Y, Jin X, Mao X, Li D (2018) TGFβ signaling controls intrahepatic bile duct development may through regulating the Jagged1-Notch-Sox9 signaling axis. J Cell Physiol 233:5780
Xiaobao F, Qiannan Z, Shuang L, Yifei L, Houqiang S, Huiping J et al (2013) Attenuation of CCl4-induced hepatic fibrosis in mice by vaccinating against TGF-β1. PLoS ONE 8:e82190
Xie G, Karaca G, Swiderska M, Michelotti GA, Krüger L, Choi SS et al (2013) Notch signaling regulates hepatic stellate cell fate by cross-talking with hedgehog signaling. Gastroenterology 144:S995
Yun-Lian L, Chia-Yu L, Chin-Wen C, Yi-Tsau H (2009) Study on antifibrotic effects of curcumin in rat hepatic stellate cells. Phytother Res 23:927–932
Zhang K, Han X, Zhang Z, Zheng L, Hu Z, Yao Q et al (2017) The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat Commun 8:144
Zhu D, He X, Duan Y, Chen J, Wang J, Sun X et al (2014) Expression of microRNA-454 in TGF-β1-stimulated hepatic stellate cells and in mouse livers infected with Schistosoma japonicum. Parasit Vectors 7:148
Acknowledgements
The authors would like to acknowledge the technical support from State Key Laboratory on Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Xinjiang Medical University. This study was supported by the Postgraduate Scientific Research Innovation Project of Xinjiang Medical University (Grant No. CXCY2018025), Xinjiang Uyghur Autonomous Region Key Laboratory Open Research Program (Grant No. 2017D04004) and Xinjiang Uyghur Autonomous Region Key Project (Grant No. 201430123).
Author information
Authors and Affiliations
Contributions
YA: Conception and design; provision of study material; collection and assembly of data; Manuscript writing and revision. MY: Collection and assembly of data; manuscript writing and revision. WL: Data Collection. TT: Provision of study material; collection and assembly of data. AS: Provision of study material; Collection and assembly of data. AM and G: Data collection. AA: Provision of study material. HW: Provision of study material; collection and assembly of data. AT: Conception and design; administrative support. YSS: Conception and design; administrative support. WH: Conception and design; administrative support; Final approval of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aimaiti, Y., Yusufukadier, M., Li, W. et al. TGF-β1 signaling activates hepatic stellate cells through Notch pathway. Cytotechnology 71, 881–891 (2019). https://doi.org/10.1007/s10616-019-00329-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-019-00329-y