Abstract
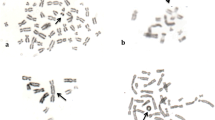
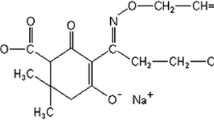
1-Naphthaleneacetamide (NAAm) is a synthetic plant growth regulator in the auxin family that is widely used in agriculture to promote the growth of numerous fruits, for root cuttings and as a fruit thinning agent. The potential genotoxic effects of NAAm were investigated in vitro by the chromosome aberrations (CAs), and cytokinesis-block micronucleus assays in human peripheral blood lymphocytes (PBLs) for the first time. The human PBLs were treated with 20, 40, 80, and 160 µg/mL of NAAm for 24 and 48 h. The results of this study showed that NAAm significantly induced the formation of structural CA and MN for all concentrations (20, 40, 80 and 160 µg/mL) and treatment periods (24 and 48 h) when compared with the negative and the solvent control. In addition, the higher concentrations of NAAm (80 and 160 µg/mL) caused a statistically significant increase in nuclear bud (NBUD) formation for both 24 and 48 h treatment times. With regard to the cell cycle kinetics, at all the tested concentrations, NAAm caused a statistically significant reduction in the mitotic index (MI) only for 48 h treatment period and also in the nuclear division index (NDI) for both 24 and 48 h treatment periods as compared to the control groups. The reductions in the MI and NDI occured in a concentration-dependent manner for both treatment times. In conclusion, the present results indicate that in the tested experimental conditions, NAAm was genotoxic and cytotoxic on human PBLs in vitro.


Similar content being viewed by others
References
Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DEG, Tice R, Waters MD, Aitio A (2000) IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res 463:111–172
Amer SM, Aly FAE (2001) Genotoxic effect of 2,4-dichlorophenoxy acetic acid and its metabolite 2,4-dichlorophenol in mouse. Mutat Res 494:1–12
Ateeq B, Farah MA, Ali MN, Ahmad W (2002) Clastogenicity of pentachlorophenol, 2,4-D and butachlor evaluated by Allium root tip test. Mutat Res 514:105–113
Bukowska B (2006) Toxicity of 2,4-dichlorophenoxyacetic acid-molecular mechanisms. Pol J Environ Stud 15:365–374
Cenkci S, Yıldız M, Ciğerci İH, Bozdağ A, Terzi H, Terzi ESA (2010) Evaluation of 2,4-D and Dicamba genotoxicity in bean seedlings using comet and RAPD assays. Ecotoxicol Environ Saf 73:1558–1564
Da Silva ES, Wong-Wah-Chung P, Burrows HD, Sarakha M (2013) Photochemical degradation of the plant growth regulator 2-(1-naphthyl) acetamide in aqueous solution upon UV irradiation. Photochem Photobiol 89:560–570
Di Paolo O, de Duffard AM, Duffard R (2001) In vivo and in vitro binding of by 2,4-dichlorophenoxyacetic acid to a rat liver mitochondrial protein. Chem Biol Interact 137:229–241
Eastmond DA, Tucker JD (1989) Identification of aneuploidy-inducing agents using cytokinesis-blocked human lymphocytes and an anti-kinetochore antibody. Environ Mol Mutagen 13:34–43
EFSA (European Food Safety Authority) (2011) Conclusion of the peer review of the pesticide risk assessment of the active substance 2-(1-naphthyl) acetamide (notified as 1-napthylacetamide). EFSA J 9:2020
Esparza X, Moyanoa E, Cosialls JR, Galceran MT (2013) Determination of naphthalene-derived compounds in apples by ultra-high performance liquid chromatography–tandem mass spectrometry. Anal Chim Acta 782:28–36
Evans HJ (1984) Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. In: Kilbey BJ, Legator M, Nichols W, Ramel C (eds) Handbook of mutagenicity test procedures, 2nd edn. Elsevier Science Publishers BV, Amsterdam, pp 405–427
Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455:81–95
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104
Fenech M, Bonassi S (2011) The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis 26:43–49
Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description of the scoring criteria fort he cytokinesis-block micronucleus assay using isolated human lymphocytes cultures. Mutat Res 534:65–75
Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, Norppa H, Eastmond DA, Tucker JD, Thomas P (2011) Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 26:125–132
Filkowski J, Besplug J, Burke P, Kovalchuk I, Kovalchuk O (2003) Genotoxicity of 2,4-D and dicamba revealed by transgenic Arabidopsis thaliana plants harboring recombination and point mutation markers. Mutat Res 542:23–32
Gisselson D, Björk J, Höglund M, Mertens F, Dal Cin P, Åkerman M, Mandahl N (2001) Abnormal nuclear shape in solid tumors reflects mitotic instability. Am J Pathol 158:199–206
Gisselsson D, Pettersson L, Höglund M, Heidenbland M, Gorunova L, Wiegant J, Mertens F, Dal Cin P, Mitelman F, Mandahl N (2000) Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogenecity. Proc Natl Acad Sci USA 97:5357–5362
Gonzàlez NV, Soloneski SE, Larramendy ML (2006) Genotoxicity analysis of the phenoxy herbicide dicamba in mammalian cells in vitro. Toxicol In Vitro 20:1481–1487
Gonzàlez NV, Soloneski S, Larramendy ML (2007) The chlorophenoxy herbicide dicamba and its commercial formulation banvel® induce genotoxicity and cytotoxicity in Chinese hamster ovary (CHO) cells. Mutat Res 634:60–68
Gonzàlez NV, Soloneski S, Larramendy ML (2009) Dicamba-induced genotoxicity in Chinese hamster ovary (CHO) cells is prevented by vitamin E. J Hazard Mater 163:337–343
Gonzàlez NV, Nikoloff N, Soloneski S, Larramendy ML (2011) A combination of the cytokinesis-block micronucleus cytome assay and centromeric identification for evaluation of the genotoxicity of dicamba. Toxicol Lett 207:204–212
Holland NT, Duramad P, Rothman N, Figgs LW, Blair A, Hubbard A, Smith MT (2002) Micronucleus frequency and proliferation in human lymphocytes after exposure to herbicide 2,4-dichlorophenoxyacetic acid in vitro and in vivo. Mutat Res 521:165–178
Kawashima Y, Katoh H, Nakajima S, Kozuka H, Uchiyama A (1984) Effects of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid on peroxisomal enzymes in rat liver. Biochem Pharmacol 33:241–245
Kegley SE, Hill BR, Orme S, Choi AH (2011) PAN Pesticide Database, Pesticide Action Network, North America, San Francisco, CA. http://www.pesticideinfo.org
Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, Ishidate M Jr, Kirchner S, Lorge E, Morita T, Norppa H, Surrallés J, Vanhauwaert A, Wakata A (2003) Report from the in vitro micronucleus assay working group. Mutat Res 540:153–163
Kocaman AY, Rencüzoğulları E, Topaktaş M, İstifli ES, Büyükleyla M (2011) The effects of 4-thujanol on chromosome aberrations, sister chromatid exchanges, and micronucleus in human peripheral blood lymphocytes. Cytotechnology 63:493–502
Korte C, Jalal SM (1982) 2,4-D induced clastogenicity and elevated rates of sister chromatid exchanges in cultured human lymphocytes. J Hered 73:224–226
Lindberg HK, Wang X, Järventaus H, Falck GC-M, Norppa H, Fenech M (2007) Origin of nuclear buds and micronuclei in normal and folate-deprived human lymphocytes. Mutat Res 617:33–45
Link H (2000) Significance of flower and fruit thinning on fruit quality. Planth Growth Reg 31:17–26
Linnainmaa K (1984) Induction of sister chromatid exchanges by the peroxisome proliferators 2,4-D, MCPA, and clofibrate in vivo and in vitro. Carcinogenesis 5:703–707
Mace ML Jr, Daskal Y, Wray W (1978) Scanning-electron microscopy of chromosome aberrations. Mutat Res 52:199–206
Madrigal-Bujaidar E, Hernàndez-Ceruelos A, Chamorro G (2001) Induction of sister chromatid exchanges by 2,4-dichlorophenoxyacetic acid in somatic and germ cells of mice exposed in vivo. Food Chem Toxicol 39:941–946
Mičić M, Bihari N, Mlinarič-Raščan I (2004) Influence of herbicide, 2,4-dichlorophenoxy acetic acid, on haemocyte DNA of in vivo treated mussel. J Exp Mar Biol Ecol 311:157–169
Mladinic M, Perkovic P, Zeljezic D (2009) Characterization of chromatin instabilities induced by glyphosate, terbuthylazine and carbofuran using cytome FISH assay. Toxicol Lett 189:130–137
Müller L, Sofuni T (2000) Appropriate levels of cytotoxicity for genotoxicity tests using mammalian cells in vitro. Environ Mol Mutagen 35:202–205
Paz-y-Miño C, Bustamante G, Sánchez ME, Leone PE (2002) Cytogenetic monitoring in a population occupationally exposed to pesticides in Ecuador. Environ Health Perspect 110:1077–1080
Reddy JK, Rao MS (1989) Oxidative DNA damage caused by persistent peroxisome proliferation: its role in hepatocarcinogenesis. Mutat Res 214:63–68
Rixe O, Fojo T (2007) Is cell death a critical end point for anticancer therapies or is cytostasis sufficient? Clin Cancer Res 13:7280–7287
Salopek-Sondi B, Piljac-Žegarac J, Magnus V, Kopjar N (2010) Free radical–scavenging activity and DNA damaging potential of auxins IAA and 2-methyl-IAA evaluated in human neutrophils by the alkaline comet assay. J Biochem Mol Toxicol 24:165–173
Song Y (2014) Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol 56:106–113
Tomlin CDS (2000) The pesticide manual, 12th edn. British Crop Protection Council Publisher, Farnham, p 661
Tuschl H, Schwab C (2003) Cytotoxic effects of the herbicide 2,4-dichlorophenoxyacetic acid in HepG2 cells. Food Chem Toxicol 41:385–393
USEPA (Environmental Protection Agency from United States of America) (2007) 738-R-07-07017 (October 2007) Registration eligibility decision (RED) for Naphthaleneacetic acid, its salts, ester and acetamide. EPA, USA
Wolff S (1982) Chromosome aberrations, sister chromatid exchanges, and the lesions that produce them. In: Wolf S (ed) Sister chromatid exchange. John Wiley & Sons Inc., New York, pp 41–57
Zeljezic D, Garaj-Vrhovac V (2004) Chromosomal aberrations, micronuclei and nuclear buds induced in human lymphocytes by 2,4-dichlorophenoxyacetic acid pesticide formulation. Toxicology 200:39–47
Acknowledgments
This study was funded by Mustafa Kemal University Research Fund (project code: 281).
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kocaman, A.Y., Güven, B. In vitro genotoxicity assessment of the synthetic plant growth regulator, 1-naphthaleneacetamide. Cytotechnology 68, 947–956 (2016). https://doi.org/10.1007/s10616-015-9847-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-015-9847-z