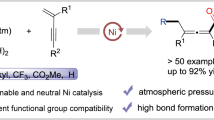
A series of 1,3,4-thiadiazol-2-amines with substituents containing SO2 group located at position 5 were obtained in good yields (63–81%) by cyclization of aryl(benzyl)sulfonylacetic or aryl(benzyl)sulfonylpropionic acids with thiosemicarbazide in refluxing POCl3.
Similar content being viewed by others
References
(a) Myznikov, L. V.; Vorona, S. V.; Zevatskii, Y. E. Chem. Heterocycl. Compd. 2021, 57, 224. (b) Makhova, N. N.; Belen'kii, L. I.; Gazieva, G. A.; Dalinger, I. L.; Konstantinova, L. S.; Kuznetsov, V. V.; Kravchenko, A. N.; Krayushkin, M. M.; Rakitin, O. A.; Starosotnikov, A. M.; Fershtat, L. L.; Shevelev, S. A.; Shirinian, V. Z.; Yarovenko, V. N. Russ. Chem. Rev. 2020, 89, 55. (c) Lipunova, G. N.; Fedorchenko, T. G.; Tsmokalyuk, A. N.; Chupakhin O. N. Russ. Chem. Bull. 2020, 69, 1203. (d) Belen'kii, L. I.; Gazieva, G. A.; Evdokimenkova, Y. B.; Soboleva, N. O. Adv. Heterocycl. Chem. 2022, 136, 225. (e) Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. Chem. Rev. 2014, 114, 5572.
Haider, S.; Alam, M. S.; Hamid, H. Eur. J. Med. Chem. 2015, 92, 156.
Anthwal, T.; Nain, S. Front. Chem. 2022, 9, 671212.
Gowda, K.; Swarup, H. A.; Nagarakere, S. C.; Rangappa, S.; Kanchugarkoppal, R. S.; Kempegowda, M. Synth. Commun. 2020, 50, 1528.
Wu, Q.; Cai, H.; Yuan, T.; Li, S.; Gan, X.; Song, B. Bioorg. Med. Chem. Lett. 2020, 30, 127113.
Gür, M.; Yerlikaya, S.; Sener, N.; Özkınalı, S.; Baloglu, M. C.; Gökçe, H.; Altunoglu, Y. C.; Demir, S.; Sener, I. J. Mol. Struct. 2020, 1219, 128570.
Miguet, L.; Zervosen, A.; Gerards, T.; Pasha, F. A.; Luxen, A.; Distèche-Nguyen, M.; Thomas, A. J. Med. Chem. 2009, 52, 5926.
(a) Dong, J.; Pei, Q.; Wang, P.; Ma, Q.; Hu, W. Arabian J. Chem. 2022, 15, 103712. (b) Serban, G. Molecules 2020, 25, 942.
Sravya, G.; Yamini, G.; Padmavathi, V.; Padmaja, A. Eur. J. Med. Chem. 2016, 122, 647.
(a) Mishra, P.; Jatav, V.; Kashaw, S. K. J. Indian Chem. Soc. 2006, 83, 1165. (b) Epishina, E. A.; Kulikov, A. S.; Ignat'ev, N. V.; Schulte, M.; Makhova, N. N. Mendeleev Commun. 2011, 21, 331. (c) Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Šiugždaitė, J.; Mickevičius, V.; Beresnevičius, Z. J. Res. Chem. Intermed. 2016, 42, 4459.
Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, Ş.; Aktay, G.; Özalp, M. Bioorg. Med. Chem. 2007, 15, 5738.
(a) Golovlyova, S. M.; Moskvichev, Yu. A.; Alov, E. M.; Kobylinsky, D. B.; Ermolaeva, V. V. Chem. Heterocycl. Compd. 2001, 37, 1102. (b) Kukaniev, M. A.; Osimov, M. D.; Sangov, Z. G.; Safarov, S. S.; Karimov, M. B.; Radjabov, T. R. Chem. Heterocycl. Compd. 2008, 44, 882.
Petkevich, S. K.; Zhukovskaya, N. А.; Dikusar, E. А.; Akishina, E. А.; Kurman, P. V.; Nikitina, E. V.; Zaytsev, V. P.; Potkin, V. I. Chem. Heterocycl. Compd. 2021, 57, 594.
Palaska, E.; Şahin, G.; Kelicen, P.; Durlu, N. T.; Altinok, G. Farmaco 2002, 57, 101.
(a) Abu-Hashem, A. A. J. Heterocycl. Chem. 2021, 58, 74. (b) Muğlu, H.; Yakan, H.; Shouaib, H. A. J. Mol. Struct. 2020, 1203, 127470.
Shainyan, B. A.; Indyukova, L. N.; Kalikhman, I. D.; Mirskova, A. N. Zh. Org. Khim. 1986, 22, 639.
(a) Serkov, S. A.; Sigai, N. V.; Kostikova, N. N.; Bulatov, P. V.; Epishina, M. A. Pharm. Chem. J. 2014, 48, 20. (b) Gazieva, G. A.; Anikina, L. V.; Nechaeva, T. V.; Pukhov, S. A.; Karpova, T. B.; Popkov, S. V.; Nelyubina, Yu. V.; Kolotyrkina, N. G.; Kravchenko, A. N. Eur. J. Med. Chem. 2017, 140, 141. (c) Gazieva, G. A.; Nechaeva, T. V.; Kostikova, N. N.; Sigay, N. V.; Serkov, S. A.; Popkov, S. V. Russ. Chem. Bull. 2018, 67, 1059. (d) Izmest'ev, A. N.; Gazieva, G. A.; Kolotyrkina, N. G.; Daeva, E. D.; Kravchenko, A. N. Chem. Heterocycl. Compd. 2020, 56, 1569. (e) Serkov, S. А.; Es'kova, M. А.; Sigay, N. V.; Kostikova, N. N.; Volkhina, T. N.; Kolotyrkina, N. G.; Gazieva, G. A. Chem. Heterocycl. Compd. 2021, 57, 646. (f) Izmest'ev, A. N.; Gazieva, G. A.; Anikina, L. V.; Pukhov, S. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N. New J. Chem. 2021, 45, 12271. (g) Serkov, S. А.; Sigay, N. V.; Kostikova, N. N.; Fedorov, A. E.; Gazieva, G. A. Russ. Chem. Bull. 2022, 71, 1801.
Abdel-wahab, A.-M. A.; El-khawaga, A. M.; El-zohry, M. F.; Khalaf, A. A. Phosphorus, Sulfur Relat. Elem. 1984, 19, 31.
(a) Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing: Twenty Fifth Informational Supplement; CLSI Document M100-S25; CLSI: Wayne, PA, 2015. (b) CLSI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard; 10th ed.; CLSI document M07-A10; CLSI: Wayne, PA, 2015. (c) CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard; 3rd ed.; CLSI document M27–A3; CLSI: Wayne, PA, 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(10), 514–517
Supplementary Information
ESM 1
(PDF 2110 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Serkov, S.A., Sigay, N.V., Kostikova, N.N. et al. Synthesis of S-substituted 5-sulfonylmethyl(ethyl)-1,3,4-thiadiazol-2-amines. Chem Heterocycl Comp 58, 514–517 (2022). https://doi.org/10.1007/s10593-022-03121-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03121-7