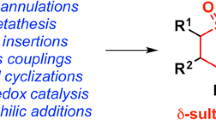
γ-Sultones condensed with the homoadamantane polycycle were synthesized by sulfonation of 1-(adamant-1-yl)-substituted olefins. The formation of γ-sultones containing a substituent at the α-position to the sulfonyl group occurs stereospecifically: 1Н and 13С NMR data support the formation of only the threo diastereomer.






Similar content being viewed by others
Notes
Ad – signals of the homoadamantane (adamantane in the spectrum of compound 2е) fragment.
References
(a) Erdmann, H. Justus Liebigs Ann. Chem. 1888, 247, 306. (b) Mustafa, A. Chem. Rev. 1954, 54, 195. (c) Buglass, A. J.; Tillet, J. G. In The Chemistry of Sulfonic Acids, Esters, and their Derivatives; Patai, S.; Rappoport, Z., Eds.; John Wiley & Sons: New York, 1991, p. 789. (d) Mondal, S. Chem. Rev. 2012, 112, 5339.
(a) Metz, P. J. Prakt. Chem. 1998, 340, 1. (b) Bequette, J. P.; Jungong, C. S.; Novikov, A. V. Tetrahedron Lett. 2009, 50, 6963. (c) Enders, D.; Iffland, D. Synthesis 2007, 12, 1837. (d) Ewas, A. M. M.; Dawood, K. M.; Spinde, K.; Wang, Y.; Jäger, A.; Metz, P. Synlett 2009, 11, 1773. (e) Merten, J.; Hennig, A.; Schwab, P.; Fröhlich, R.; Tokalov, S. V.; Gutzeit, H. O.; Metz, P. Eur. J. Org. Chem. 2006, 5, 1144.
Roberts, D. W.; Williams, D. L. Tetrahedron 1987, 43, 1027.
(a) Solas, D.; Wolinsky, J. J. Org. Chem. 1983, 48, 1988. (b) Wang, Y.; Bernsmann, H.; Gruner, M.; Metz, P. Tetrahedron Lett. 2001, 42, 7801. (c) Metz, P.; Stölting, J.; Läge, M.; Krebs, B. Angew. Chem., Int. Ed. Engl. 1994, 33, 2195.
(a) De Castro, S.; Lobatón, E.; Pérez-Pérez, M.-J.; San-Félix, A.; Cordeiro, A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J.; Camarasa, M.-J.; Velázquez, S. J. Med. Chem. 2005, 48, 1158. (b) De Castro, S.; García-Aparicio, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Camarasa, M.-J.; Velázquez, S. J. Med. Chem. 2009, 52, 1582. (c) De Castro, S.; Peromingo, M. T.; Naesens, L.; Andrei, G.; Snoeck, R.; Balzarini, J.; Velázquez, S.; Camarasa, M.-J. J. Med. Chem. 2008, 51, 5823. (d) Rodríguez-Barrios, F.; Pérez, C.; Lobatón, E.; Velázquez, S.; Chamorro, C.; San-Félix, A.; Pérez-Pérez, M.-J.; Camarasa, M.-J.; Pelemans, H.; Balzarini, J.; Gago, F. J. Med. Chem. 2001, 44, 1853. (e) Velázquez, S.; Lobatón, E.; De Clercq, E.; Koontz, D. L.; Mellors, J. W.; Balzarini, J.; Camarasa, M.-J. J. Med. Chem. 2004, 47, 3418. (f) Xu, H.-W.; Zhao, L.-J.; Liu, H.-F.; Zhao, D.; Luo, J.; Xie, X.-P.; Liu, W.-S.; Zheng, J.-X.; Dai, G.-F.; Liu, H.-M.; Liu, L.-H.; Liang, Y.-B. Bioorg. Med. Chem. Lett. 2014, 24, 2388.
(a) Kovalev, V. V.; Shokova, E. A. Zh. Org. Khim. 1988, 24, 738. (b) Pérez-Pérez, M.-J.; Balzarini, J.; Hosoya, M.; De Clercq, E.; Camarasa, M.-J. Bioorg. Med. Chem. Lett. 1992, 2, 647. (c) Leonova, M. V.; Baimuratov, M. R.; Klimochkin, Yu. N. Russ. J. Org. Chem. 2015, 51, 26. [Zh. Org. Khim. 2015, 51, 33.] (d) Aslanov, L. A.; Tafeenko, V. A.; Kovalev, V. V.; Shokova, É. A. J. Struct. Chem. (USSR) 1989, 30, 933. [Zh. Strukt. Khim. 1989, 30(6), 79.]
(a) Roberts, D. W.; Jackson, P. S.; Saul, C. D.; Clemett, C. J. Tetrahedron Lett. 1987, 28, 3383. (b) Bordwell, F. G.; Chapman, R. D.; Osborne, C. E. J. Am. Chem. Soc. 1959, 81, 2002. (c) Terent'ev, A. P.; Potapov, V. M.; Dem'yanovich, V. M. Zh. Obshch. Khim. 1959, 29, 949. (d) Zyk, N. V.; Beloglazkina, E. K.; Zefirov, N. S. Zh. Org. Khim. 1995, 31, 1283.
(a) Leonova, M. V.; Baimuratov, M. R.; Golovin, E. V.; Klimochkin, Yu. N. Russ. J. Org. Chem. 2014, 50, 183. [Zh. Org. Khim. 2014, 50, 194.] (b) Leonova, M. V.; Baimuratov, M. R.; Klimochkin, Yu. N. Russ. J. Org. Chem. 2014, 50, 1268. [Zh. Org. Khim. 2014, 50, 1285.] (c) Baimuratov, M. R.; Leonova, M. V.; Klimochkin, Yu. N. Russ. J. Gen. Chem. 2014, 84, 632. [Zh. Obshch. Khim. 2014, 84, 552.]
(a) Klimochkin, Yu. N.; Korzhev, I. R.; Vologin, M. F.; Bagrii, E. I. Petroleum Chemistry 2001, 41, 30. [Neftekhimiya 2001, 41, 30.] (b) Stetter, H.; Rauscher, E. Chem. Ber. 1960, 93, 2054. (c) Sai, M.; Yorimitsu, H.; Oshima, K. Bull. Chem. Soc. Jpn. 2009, 82, 1194.
(a) Olah, G. A. Angew. Chem., Int. Ed. Engl. 1973, 12, 173. (b) Lenoir, D.; Schleyer, P. von R. Chem. Commun. 1970, 941. (c) Lenoir, D.; Schleyer, P. von R. Angew. Chem. 1971, 83, 918. (d) Leonova, M. V.; Klimochkin, Yu. N.; Moiseev, I. K. Russ. J. Org. Chem. 1998, 34, 494. [Zh. Org. Khim. 1998, 34, 528.] (e) Krasutskii, P. A.; Semenova, I. G.; Safronova, E. E. Russ. J. Org. Chem. 1992, 28, 1827. [Zh. Org. Khim. 1992, 28, 2268.] (f) Lucchini, V.; Modena, G.; Pasquato, L. J. Am. Chem. Soc. 1991, 113, 6600.(g) Duddeck, H.; Rosenbaum, D. J. Org. Chem. 1991, 56, 1707. (h) Allen, A. D.; Krishnamurti, R.; Surya Prakash, G. K.; Tidwell, T. T. J. Am. Chem. Soc. 1990, 112, 1291.
(a) Bakker, B. H.; Cerfontain, H. Tetrahedron Lett. 1987, 28, 1699. (b) Bakker, B. H.; Cerfontain, H. Tetrahedron Lett. 1987, 28, 1703.
Roberts, D. W. Org. Proc. Res. Dev. 1998, 2, 194.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Spek, A. L. Acta Crystallogr., Sect. A: Found. Crystallogr. 1990, A46, 34.
This work was supported by funding from the Ministry of Education and Science of the Russian Federation within the framework of the Project part of the State Assignment № 4.1440.2014/К as well as by funding from the Russian Foundation for Basic Research (grant 15-43-02536 r_povolzhye_a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(6), 582–585
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2813 kb)
Rights and permissions
About this article
Cite this article
Baimuratov, M.R., Leonova, M.V., Rybakov, V.B. et al. Synthesis of γ-sultones based on conversion of adamantane-type olefins. Chem Heterocycl Comp 51, 582–585 (2015). https://doi.org/10.1007/s10593-015-1740-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1740-3