Abstract
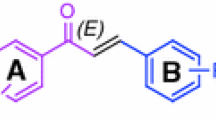
An efficient method has been developed for the first time for the synthesis of α,β-unsaturated aldehydes with an (E)-trisubstituted C=C double bond by oxidation of mixtures of regio- and stereoisomeric allyl bromides without their preliminary separation. The substrates are available via ring opening of 1,2-disubstituted cyclopropyl sulfonates. Allyl bromides containing additional functional groups were oxidized with N-methylmorpholine N-oxide more effectively. The reactions were carried out in solvents favoring nucleophilic substitution, and elevated temperature and prolonged reaction increased the fraction of the E isomer and reduced the amount of isomeric ketone.





Similar content being viewed by others
REFERENCES
Wichard, T., Göbel, C., Feussner, I., and Pohnert, G., Angew. Chem., Int. Ed., 2005, vol. 44, p. 158. https://doi.org/10.1002/anie.200460686
Das, B., Banerjee, J., Chowdhury, N., Majhi, A., and Mahender, G., Helv. Chim. Acta, 2006, vol. 89, p. 876. https://doi.org/10.1002/hlca.200690090
Taylor, R.E. and Paquette, W.D., Org. Lett., 2004, vol. 6, p. 103. https://doi.org/10.1021/ol0361397
Smith, A.B., Basu, K., and Bosanac, T., J. Am. Chem. Soc., 2007, vol. 129, p. 14872. https://doi.org/10.1021/ja077569l
Dawson, G.W., Pickett, J.A., and Smiley, D.W.M., Bioorg. Med. Chem., 1996, vol. 4, p. 351. https://doi.org/10.1016/0968-0896(96)00012-0
Escher, I. and Glorius, F., Science of Synthesis, Brückner, R., Ed., Stuttgart: Thieme, 2007, vol. 25, p. 733.
Olah, G.A. and Arvanaghi, M., Angew. Chem., Int. Ed. Engl., 1981, vol. 20, p. 878. https://doi.org/10.1002/anie.198108781
Crimmins, M.T. and King, B.W., J. Am. Chem. Soc., 1998, vol. 120, p. 9084. https://doi.org/10.1021/ja9817500
Sirasani, G., Paul, T., and Andrade, R.B., Tetrahedron, 2011, vol. 67, p. 2197. https://doi.org/10.1016/j.tet.2011.01.080
Wittig, G. and Reiff, H., Angew. Chem., Int. Ed. Engl., 1968, vol. 7, p. 7. https://doi.org/10.1002/anie.196800071
Corey, E.J., Enders, D., and Bock, M.G., Tetrahedron Lett., 1976, vol. 17, p. 7. https://doi.org/10.1016/S0040-4039(00)71308-6
Duhamel, L., Gralak, J., and Bouyanzer, A., Tetrahedron Lett., 1993, vol. 34, p. 7745. https://doi.org/10.1016/S0040-4039(00)61554-X
Wollenberg, R.H., Albizati, K.F., and Peries, R., J. Am. Chem. Soc., 1977, vol. 99, p. 7365. https://doi.org/10.1021/ja00464a051
Zhou, G., Hu, Q.-Y., and Corey, E.J., Org. Lett., 2003, vol. 5, p. 3979. https://doi.org/10.1021/ol035542a
Nagamitsu, T., Takano, D., Fukuda, T., Otoguro, K., Kuwajima, I., Harigaya, Y., and Omura, S., Org. Lett., 2004, vol. 6, p. 1865. https://doi.org/10.1021/ol049356w
Johnson, J.R., Cuny, G.D., and Buchwald, S.L., Angew. Chem., Int. Ed. Engl., 1995, vol. 34, p. 1760. https://doi.org/10.1002/anie.199517601
Bruch, A., Gebert, A., and Breit, B., Synthesis, 2008, vol. 2008, p. 2169. https://doi.org/10.1055/s-2008-1067140
Sato, T., Okazaki, H., Otera, J., and Nozaki, H., Tetrahedron Lett., 1988, vol. 29, p. 2979. https://doi.org/10.1016/0040-4039(88)85063-9
Babler, J.H., Coghlan, M.J., Feng, M., and Fries, P., J. Org. Chem., 1979, vol. 44, p. 1716. https://doi.org/10.1021/jo01324a030
D’Aniello, F., Mattii, D., and Taddei, M., Synlett, 1993, vol. 1993, p. 119. https://doi.org/10.1055/s-1993-22369
Mukaiyma, S., Inanaga, J., and Yamaguchi, M., Bull. Chem. Soc. Jpn., 1981, vol. 54, p. 2221. https://doi.org/10.1246/bcsj.54.2221
Suzuki, S., Onishi, T., Fujita, Y., Misawa, H., and Otera, J., Bull. Chem. Soc. Jpn., 1986, vol. 59, p. 3287. https://doi.org/10.1246/bcsj.59.3287
Hayashi, T., Tetrahedron Lett., 1990, vol. 31, p. 4155. https://doi.org/10.1016/S0040-4039(00)97568-3
Ganem, B. and Godfrey, A., Tetrahedron Lett., 1990, vol. 31, p. 4825. https://doi.org/10.1016/S0040-4039(00)97742-6
Griffith, W.P., Jolliffe, J.M., Ley, S.V., Springhorn, K.F., and Tiffin, P.D., Synth. Commun., 1992, vol. 22, p. 967. https://doi.org/10.1080/00397919208021328
Chandrasekhar, S. and Sridhar, M., Tetrahedron Lett., 2000, vol. 41, p. 5423. https://doi.org/10.1016/S0040-4039(00)00874-1
Chen, D.X., Ho, C.M., Rudy Wu, Q.Y., Wu, P.R., Wong, F.M., and Wu, W., Tetrahedron Lett., 2008, vol. 49, p. 4147. https://doi.org/10.1016/j.tetlet.2008.04.124
Cardillo, G., Orena, M., and Sandri, S., Chem. Commun., 1976, no. 6, p. 190. https://doi.org/10.1039/C39760000190
Cardillo, G., Orena, M., and Sandri, S., Tetrahedron Lett., 1976, vol. 17, p. 3985. https://doi.org/10.1016/S0040-4039(00)92554-1
Suzuki, S., Onishi, T., Fujita, Y., and Otera, J., Synth. Commun., 1985, vol. 15, p. 1123. https://doi.org/10.1080/00397918508077254
Krohnke, F., Angew. Chem., Int. Ed. Engl., 1963, vol. 2, p. 380. https://doi.org/10.1002/anie.196303801
Karamé, I., Jahjah, M., Messaoudi, A., Tommasino, M.L., and Lemaire, M., Tetrahedron: Asymmetry, 2004, vol. 15, p. 1569. https://doi.org/10.1016/j.tetasy.2004.03.023
Das, S., Panigrahia, A.K., and Maikap, G.C., Tetrahedron Lett., 2003, vol. 44, p. 1375. https://doi.org/10.1016/S0040-4039(02)02885-X
Li, C., Angew. Chem., Int. Ed., 2003, vol. 42, p. 5063. https://doi.org/10.1002/anie.200351902
Moorthy, J.N., Singhal, N., and Senapati, K., Tetrahedron Lett., 2006, vol. 47, p. 1757. https://doi.org/10.1016/j.tetlet.2006.01.039
Tang, J., Zhu, J., Shen, Z., and Zhang, Y., Tetrahedron Lett., 2007, vol. 48, p. 1919. https://doi.org/10.1016/j.tetlet.2007.01.084
Sofiyev, V., Navarro, G., and Trauner, D., Org. Lett., 2008, vol. 10, p. 149. https://doi.org/10.1021/ol702806v
Kozyrkov, Yu.Yu. and Kulinkovich, O.G., Synlett, 2002, vol. 2002, p. 443. https://doi.org/10.1055/s-2002-20461
Kananovich, D.G., Hurski, A.L., and Kulinkovich, O.G., Tetrahedron Lett., 2007, vol. 48, p. 8424. https://doi.org/10.1016/j.tetlet.2007.09.172
Gatsak, E.L. and Kozyr’kov, Yu.Yu., Sbornik rabot 69-i nauchnoi konferentsii studentov i aspirantov Belorusskogo gosudarstvennogo universiteta (A Collection of Papers Presented at the 69th Scientific Conf. of Students and Postgraduate Students of Belarusian State University), May 14–17, 2012, Minsk, p. 307.
Podunavac, M., Lacharity, J., Jones, K.E., and Zakarian, A., Org. Lett., 2018, vol. 20, p. 4867. https://doi.org/10.1021/acs.orglett.8b02011
Rountree, S.M., Taylor, S.F.R., Hardacre, C., Lagunas, M.C., and Davey, P.N., Appl. Catal., A, 2014, vol. 486, p. 94. https://doi.org/10.1016/j.apcata.2014.08.032
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 10, pp. 1357–1370 https://doi.org/10.31857/S0514749221100013.
Rights and permissions
About this article
Cite this article
Masyuk, V.S., Kozyrkov, Y.Y. & Mineeva, I.V. Synthesis of α,β-Unsaturated Aldehydes with an (E)-Trisubstituted Double Bond via Ring Opening of Cyclopropanols. Russ J Org Chem 57, 1563–1574 (2021). https://doi.org/10.1134/S1070428021100018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021100018