Abstract
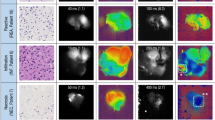
5-ALA fluorescence-guided surgery (FGS) is a major advance in neuro-oncological surgery. So far, Protoporphyrin IX (PpIX)-fluorescence has been observed in about half of cerebral metastases resected with routinely equipped microscopes during 5-ALA FGS. The aim of the present pilot study was to quantify PpIX-induced fluorescence of cerebral metastases with a spectrometer. We hypothesize that non-fluorescing metastases under the operating microscope may have spectrometrically measurable levels of fluorescence. A second aim was to analyze correlations between quantified 5-ALA fluorescence and histology or primary tumor type, respectively. Standard FGS was performed in all patients. The fluorescence intensity of the metastasis was semi-quantitatively determined in vivo by a senior surgeon using a special surgical microscope equipped for FGS. A systematic spectrometric ex vivo evaluation of tumor specimens and PpIX-induced fluorescence was performed using a spectrometer connected by optic fibers to a handheld probe. Quantification of 5-ALA-derived fluorescence was measured in a standardized manner with direct contact between mini-spectrometer and metastasis. The difference between the maximum PpIX-fluorescence at 635 nm and the baseline fluorescence was defined as the PpIX fluorescence intensity of the metastasis and given in arbitrary units (AU). Diagnosis of a cerebral metastasis was confirmed by histopathological analysis. A total of 29 patients with cerebral metastases were included. According to neuropathological analysis, 11 patients suffered from non-small cell lung cancer, 10 patients from breast cancer, 6 patients from cancer originating in the gastro-intestinal tract, 1 patient suffered from a malignant melanoma and one patient from renal cancer. The mean age was 63 years (37–81 years). 15 patients were female, 14 patients male. 13 cerebral metastases were considered as ALA-positive by the surgeon. In nine metastases, 5-ALA fluorescence was not visible to the naked eye and could only be detected using the spectrometer. The threshold for an ALA signal rated as “positive” by the surgeon was PpIX fluorescence above 1.1 × 106 AU. The mean PpIX fluorescence of all analyzed cerebral metastases was 1.29 × 106 ± 0.23 × 106 AU. After quantification, we observed a significant difference between the mean 5-ALA-derived fluorescence in NSCLC and breast cancer metastases (Mean Diff: − 1.2 × 106; 95% CI of difference: − 2.2 × 106 to − 0.15 × 106; Šidák-adjusted p = 0.026). In our present pilot series, about half of cerebral metastases showed a 5-ALA fluorescence invisible to the naked eye. Over 50% of these non-fluorescent metastases show a residual 5-ALA fluorescence which can be detected and quantified using a spectrometer. Moreover, the quantified 5-ALA signal significantly differed with respect to the primary tumor of the corresponding cerebral metastasis. Further studies should evaluate the predictive value of the 5-ALA signal and if a quantified 5-ALA signal enables a reliable intraoperative differentiation between residual tumor tissue and edematous brain—in particular in metastases with a residual fluorescence signal invisible to the naked eye.


Similar content being viewed by others
Abbreviations
- 5-ALA:
-
5-Aminolevulinic acid
- AU:
-
Arbitrary unit
- PpIX:
-
Protoporphyrin IX
- FGS:
-
Fluorescence guided surgery
- NSCLC:
-
Non-small-cell lung cancer
- SCLC:
-
Small-cell lung cancer
- nm:
-
Nanometer
- CI:
-
Confidence interval
- p:
-
p-value
- U.S.:
-
United States of America
- PFS:
-
Progression-free survival
- min:
-
Minute
- e.g.:
-
Exempli gratia
- EGFR:
-
Epidermal growth factor receptor
- ALK:
-
Anaplastic lymphoma kinase
- HER2/neu:
-
Human epidermal growth factor receptor 2
References
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5–14. https://doi.org/10.1007/s11060-004-8093-6
Patchell RA (2003) The management of brain metastases. Cancer Treat Rev 29:533–540
Aronson SM, Garcia JH, Aronson BE (1964) Metastatic neoplasms of the brain: their frequency in relation to age. Cancer 17:558–563
Chason JL, Walker FB, Landers JW (1963) Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer 16:781–787
Shojania KG, Burton EC, McDonald KM, Goldman L (2003) Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA 289:2849–2856. https://doi.org/10.1001/jama.289.21.2849
Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, Perez CA, Hendrickson FR (1980) The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 6:1–9
Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, Grosu AL, Guckenberger M (2014) Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190:521–532. https://doi.org/10.1007/s00066-014-0648-7
Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, Bendszus M, Reijneveld JC, Dhermain F, French P, Marosi C, Watts C, Oberg I, Pilkington G, Baumert BG, Taphoorn MJB, Hegi M, Westphal M, Reifenberger G, Soffietti R, Wick W, European Association for Neuro-Oncology Task Force on G (2017) European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18:e315–e329. https://doi.org/10.1016/S1470-2045(17)30194-8
Stummer W, Kamp MA (2009) The importance of surgical resection in malignant glioma. Curr Opin Neurol 22:645–649. https://doi.org/10.1097/WCO.0b013e3283320165
Kamp MA, Dibue M, Santacroce A, Zella SM, Niemann L, Steiger HJ, Rapp M, Sabel M (2013) The tumour is not enough or is it? Problems and new concepts in the surgery of cerebral metastases. Ecancermedicalscience 7:306. https://doi.org/10.3332/ecancer.2013.306
Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, Marosi C, Metellus P, Radbruch A, Villa Freixa SS, Brada M, Carapella CM, Preusser M, Le Rhun E, Ruda R, Tonn JC, Weber DC, Weller M (2017) Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 19:162–174. https://doi.org/10.1093/neuonc/now241
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134–141. https://doi.org/10.1200/JCO.2010.30.1655
Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, Settle S, Prabhu SS, Lang FF, Levine N, McGovern S, Sulman E, McCutcheon IE, Azeem S, Cahill D, Tatsui C, Heimberger AB, Ferguson S, Ghia A, Demonte F, Raza S, Guha-Thakurta N, Yang J, Sawaya R, Hess KR, Rao G (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048. https://doi.org/10.1016/S1470-2045(17)30414-X
Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS, Zo JI, Lee SH (2009) Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg 110:730–736. https://doi.org/10.3171/2008.8.JNS08448
Kamp MA, Dibue-Adjei M, Cornelius JF, Slotty PJ, Steiger HJ, Ahmadi SA, Rapp M, Sabel M (2018) Is it all a matter of size? Impact of maximization of surgical resection in cerebral tumors. Neurosurg Rev. https://doi.org/10.1007/s10143-018-0963-z
Kamp MA, Dibue M, Niemann L, Reichelt DC, Felsberg J, Steiger HJ, Szelenyi A, Rapp M, Sabel M (2012) Proof of principle: supramarginal resection of cerebral metastases in eloquent brain areas. Acta Neurochir (Wien) 154:1981–1986. https://doi.org/10.1007/s00701-012-1463-5
Kamp MA, Rapp M, Buhner J, Slotty PJ, Reichelt D, Sadat H, Dibue-Adjei M, Steiger HJ, Turowski B, Sabel M (2015) Early postoperative magnet resonance tomography after resection of cerebral metastases. Acta Neurochir (Wien) 157:1573–1580. https://doi.org/10.1007/s00701-015-2479-4
Kamp MA, Rapp M, Slotty PJ, Turowski B, Sadat H, Smuga M, Dibue-Adjei M, Steiger HJ, Szelenyi A, Sabel M (2015) Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir (Wien) 157:905–910. https://doi.org/10.1007/s00701-015-2405-9 (discussion 910–901)
Kamp MA, Slotty PJ, Cornelius JF, Steiger HJ, Rapp M, Sabel M (2018) The impact of cerebral metastases growth pattern on neurosurgical treatment. Neurosurg Rev 41:77–86. https://doi.org/10.1007/s10143-016-0760-5
Olesrud IC, Schulz MK, Marcovic L, Kristensen BW, Pedersen CB, Kristiansen C, Poulsen FR (2019) Early postoperative MRI after resection of brain metastases-complete tumour resection associated with prolonged survival. Acta Neurochir (Wien) 161:555–565. https://doi.org/10.1007/s00701-019-03829-0
Patel AJ, Suki D, Hatiboglu MA, Abouassi H, Shi W, Wildrick DM, Lang FF, Sawaya R (2010) Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg 113:181–189. https://doi.org/10.3171/2009.11.JNS09659
Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD (2008) Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 108:248–257. https://doi.org/10.3171/JNS/2008/108/2/0248
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013. https://doi.org/10.3171/jns.2000.93.6.1003
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. https://doi.org/10.1016/s1470-2045(06)70665-9
Cornelius JF, Kamp MA, Tortora A, Knipps J, Krause-Molle Z, Beez T, Petridis AK, Sabel M, Schipper J, Steiger HJ (2019) Surgery of small anterior skull base meningiomas by endoscopic 5-aminolevulinic acid fluorescence guidance: first clinical experience. World Neurosurg 122:e890–e895. https://doi.org/10.1016/j.wneu.2018.10.171
Cornelius JF, Placke JM, Knipps J, Fischer I, Kamp M, Steiger HJ (2017) Minispectrometer with handheld probe for 5-ALA based fluorescence-guided surgery of brain tumors: preliminary study for clinical applications. Photodiagn Photodyn Ther 17:147–153. https://doi.org/10.1016/j.pdpdt.2016.12.007
Cornelius JF, Slotty PJ, Kamp MA, Schneiderhan TM, Steiger HJ, El-Khatib M (2014) Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas—with special regard to high-grade tumors. Photodiagn Photodyn Ther 11:481–490. https://doi.org/10.1016/j.pdpdt.2014.07.008
Cornelius JF, Slotty PJ, Stoffels G, Galldiks N, Langen KJ, Steiger HJ (2013) 5-Aminolevulinic acid and (18)F-FET-PET as metabolic imaging tools for surgery of a recurrent skull base meningioma. J Neurol Surg B Skull Base 74:211–216. https://doi.org/10.1055/s-0033-1342918
Eicker SO, Floeth FW, Kamp M, Steiger HJ, Hanggi D (2013) The impact of fluorescence guidance on spinal intradural tumour surgery. Eur Spine J 22:1394–1401. https://doi.org/10.1007/s00586-013-2657-0
Evers G, Kamp M, Warneke N, Berdel WE, Sabel M, Stummer W, Ewelt C (2016) 5-aminolaevulinic acid-induced fluorescence in primary central nervous system lymphoma. World Neurosurg. https://doi.org/10.1016/j.wneu.2016.11.011
Knipps J, Beseoglu K, Kamp M, Fischer I, Felsberg J, Neumann LM, Steiger HJ, Cornelius JF (2017) Fluorescence behavior and dural infiltration of meningioma analyzed by 5-aminolevulinic acid-based fluorescence: operating microscope versus mini-spectrometer. World Neurosurg 108:118–127. https://doi.org/10.1016/j.wneu.2017.08.140
Krause Molle Z, Gierga K, Turowski B, Steiger HJ, Cornelius JF, Rapp M, Sabel M, Kamp MA (2018) 5-ALA-induced fluorescence in leptomeningeal dissemination of spinal malignant glioma. World Neurosurg 110:345–348. https://doi.org/10.1016/j.wneu.2017.10.069
Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M, Roelcke U, Fathi AR, Coluccia D, Fandino J (2014) Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 36:E10. https://doi.org/10.3171/2013.12.FOCUS13464
Millesi M, Kiesel B, Mischkulnig M, Martinez-Moreno M, Wohrer A, Wolfsberger S, Knosp E, Widhalm G (2016) Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: experience in 204 meningiomas. J Neurosurg 125:1408–1419. https://doi.org/10.3171/2015.12.JNS151513
Yamamoto J, Kitagawa T, Akiba D, Nishizawa S (2015) 5-Aminolevulinic acid-induced fluorescence in cerebellar primary central nervous system lymphoma: a case report and literature review. Turk Neurosurg 25:796–800. https://doi.org/10.5137/1019-5149.JTN.10594-14.1
Yamamoto T, Ishikawa E, Miki S, Sakamoto N, Zaboronok A, Matsuda M, Akutsu H, Nakai K, Tsuruta W, Matsumura A (2015) Photodynamic diagnosis using 5-aminolevulinic acid in 41 biopsies for primary central nervous system lymphoma. Photochem Photobiol 91:1452–1457. https://doi.org/10.1111/php.12510
Belloch JP, Rovira V, Llacer JL, Riesgo PA, Cremades A (2014) Fluorescence-guided surgery in high grade gliomas using an exoscope system. Acta Neurochir (Wien) 156:653–660. https://doi.org/10.1007/s00701-013-1976-6
Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, Wirtz CR, Konig R (2014) Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 36:E3. https://doi.org/10.3171/2013.11.FOCUS13463
Kamp MA, Grosser P, Felsberg J, Slotty PJ, Steiger HJ, Reifenberger G, Sabel M (2012) 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien) 154:223–228. https://doi.org/10.1007/s00701-011-1200-5 (discussion 228)
Piquer J, Llacer JL, Rovira V, Riesgo P, Rodriguez R, Cremades A (2014) Fluorescence-guided surgery and biopsy in gliomas with an exoscope system. Biomed Res Int 2014:207974. https://doi.org/10.1155/2014/207974
Schucht P, Beck J, Vajtai I, Raabe A (2011) Paradoxical fluorescence after administration of 5-aminolevulinic acid for resection of a cerebral melanoma metastasis. Acta Neurochir (Wien) 153:1497–1499. https://doi.org/10.1007/s00701-011-0991-8
Utsuki S, Miyoshi N, Oka H, Miyajima Y, Shimizu S, Suzuki S, Fujii K (2007) Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol 24:53–55. https://doi.org/10.1007/s10014-007-0223-3
Valdes PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan X, Tosteson TD, Hartov A, Ji S, Erkmen K, Simmons NE, Paulsen KD, Roberts DW (2011) Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg 115:11–17. https://doi.org/10.3171/2011.2.JNS101451
Widhalm G, Minchev G, Woehrer A, Preusser M, Kiesel B, Furtner J, Mert A, Di Ieva A, Tomanek B, Prayer D, Marosi C, Hainfellner JA, Knosp E, Wolfsberger S (2012) Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg Rev 35:381–391. https://doi.org/10.1007/s10143-012-0374-5 (discussion 391)
Kamp MA, Fischer I, Buhner J, Turowski B, Cornelius JF, Steiger HJ, Rapp M, Slotty PJ, Sabel M (2016) 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget. https://doi.org/10.18632/oncotarget.11488
Kamp MA, Munoz-Bendix C, Mijderwijk HJ, Turowski B, Dibue-Adjei M, von Sass C, Cornelius JF, Steiger HJ, Rapp M, Sabel M (2019) Is 5-ALA fluorescence of cerebral metastases a prognostic factor for local recurrence and overall survival? J Neurooncol 141:547–553. https://doi.org/10.1007/s11060-018-03066-y
Arjona SG, Almunia ML, Dominguez JAI, Sanchez OD, Villalonga P, Villalonga-Planells R, Lopetegui JP, Escalas JB, Barcelo AM, Doval MB (2019) Comparison of commercial 5-aminolevulinic acid (Gliolan(R)) and the pharmacy-compounded solution fluorescence in glioblastoma. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-019-03930-4
Kamp MA, Santacroce A, Zella S, Reichelt DC, Felsberg J, Steiger HJ, Cornelius JF, Sabel M (2012) Is it a glioblastoma? In dubio pro 5-ALA! Acta Neurochir (Wien) 154:1269–1273. https://doi.org/10.1007/s00701-012-1369-2
Kamp MA, Krause Molle Z, Munoz-Bendix C, Rapp M, Sabel M, Steiger HJ, Cornelius JF (2018) Various shades of red-a systematic analysis of qualitative estimation of ALA-derived fluorescence in neurosurgery. Neurosurg Rev 41:3–18. https://doi.org/10.1007/s10143-016-0745-4
Kamp MAR, Cornelius JF, Steiger HJ, Sabel M (2019) 5-Aminolaevulinic acid and brain metastases. In: Hadjipanayis CGS (ed) Fluorescence-guided neurosurgery: neuro-oncology and cerebrovascular application. Thieme, New York, Stuttgart, Delhi, Rio de Janerio, pp 39–43
Kamp MA, Krause Molle Z, Munoz-Bendix C, Rapp M, Sabel M, Steiger HJ, Cornelius JF (2016) Various shades of red-a systematic analysis of qualitative estimation of ALA-derived fluorescence in neurosurgery. Neurosurg Rev. https://doi.org/10.1007/s10143-016-0745-4
Berghoff AS, Rajky O, Winkler F, Bartsch R, Furtner J, Hainfellner JA, Goodman SL, Weller M, Schittenhelm J, Preusser M (2013) Invasion patterns in brain metastases of solid cancers. Neuro Oncol 15:1664–1672. https://doi.org/10.1093/neuonc/not112
Acknowledgements
We thank Maria Smuga and Osman Auale for their help.
Funding
The present study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. Sabel and PD Dr. Rapp work as consultants for Johnson & Johnson Company and Integra Company. Dr. Dibué-Adjei is an employee of LivaNova PLC, manufacturer of vagus nerve stimulators. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical statement
Informed consent was obtained. The present analysis was performed in accordance with the Declaration of Helsinki and with the acceptance of the local Research Ethics Committee and institutional review board (internal study number: 4266).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Knipps, J., Fischer, I., Neumann, L.M. et al. Quantification of PpIX-fluorescence of cerebral metastases: a pilot study. Clin Exp Metastasis 36, 467–475 (2019). https://doi.org/10.1007/s10585-019-09986-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-019-09986-x