Abstract
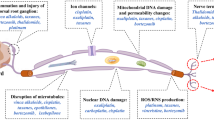
Metastatic cancers are associated with cellular oxidative stress, and during cancer chemotherapy excess drug-induced oxidative stress can limit therapeutic effectiveness and cause a number of side effects, including fatigue, nausea, vomiting, diarrhea and more serious adverse effects, such as cardiomyopathy, peripheral neuropathy, hepatotoxicity and pulmonary fibrosis. We review here the hypothesis that the acute and chronic adverse effects of cancer chemotherapy can be reduced by molecular replacement of membrane lipids and enzymatic cofactors, such as coenzyme Q10. By administering nutritional supplements with replacement molecules and antioxidants, oxidative membrane damage and reductions of cofactors in normal tissues can be reversed, protecting and restoring mitochondrial and other cellular functions and reducing chemotherapy adverse effects. Recent clinical trials using cancer and non-cancer patients with chronic fatigue have shown the benefit of molecular replacement plus antioxidants in reducing the damage to mitochondrial membranes, restoring mitochondrial electron transport function, reducing fatigue and protecting cellular structures and enzymes from oxidative damage. Molecular replacement and antioxidant administration mitigates the damage to normal tissues, such as cardiac tissue, and reduces the adverse effects of cancer therapy without reduction in therapeutic results.
Similar content being viewed by others
References
Kehrer JP (1993) Free radicals and mediators of tissue injury and disease. Crit Rev Toxicol 23:21–48
Halliwell B (1996) Oxidative stress, nutrition and health. Free Rad Res 25:57–74
Dreher D, Junod AF (1996) Role of oxygen free radicals in cancer development. Eur J Cancer 32A:30–38
Abidi S, Ali A (1999) Role of oxygen free radicals in the pathogenesis and etiology of cancer. Cancer Lett 142:1–9
Stadtman E (2002) Introduction to serial reviews on oxidatively modified proteins in aging and disease. Free Radic Biol Med 32:789
Marnett LJ (2000) Oxyradicals and DNA damage. Carcinogenesis 21:361–370
Bartsch H, Nair J (2004) Oxidative stress and lipid peroxidation-driven DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev 28:385–391
Castro L, Freeman BA (2001) Reactive oxygen species in human health and disease. Nutrition 17:295–307
Johnson TM, Yu ZX, Ferrans VJ et al (1996) Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA 93:11848–11852
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267
Barber DA, Harris SR (1994) Oxygen free radicals and antioxidants: a review. Am Pharm 34:26–35
Sun Y (1990) Free radicals, antioxidant enzymes and carcinogenesis. Free Radic Biol Med 8:583–599
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Seifried HE, McDonald SS, Anderson DE et al (2003) The antioxidant conundrum in cancer. Cancer Res 61:4295–4298
Jagetia GC, Rajanikant GK, Rao SK et al (2003) Alteration in the glutathione, glutathione peroxidase, superoxide dismutase and lipid peroxidation by ascorbic acid in the skin of mice exposed to fractionated gamma radiation. Clin Chim Acta 332:111–121
Schwartz JL (1996) The dual roles of nutrients as antioxidants and prooxidants: their effects on tumor cell growth. J Nutr 126:1221S–1227S
Aeschbach R, Loliger J, Scott BC et al (1994) Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol 32:31–36
Tanaka T (1994) Cancer chemoprevention by natural products. Oncol Rep 1:1139–1155
Prasad KN, Cole WC, Kumar B et al (2001) Scientific rationale for using high-dose multiple micronutrients as an adjunct to standard and experimental cancer therapies. J Am Coll Nutr 20:450S–453S
Toyokuni S, Okamoto K, Yodio J et al (1995) Persistent oxidative stress in cancer. FEBS Lett 358:1–3
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267
Brown NS, Bicknell R (2001) Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 3:323–327
Kline K, Yu W, Sanders BG (2004) Vitamin E and breast cancer. J Nutr 134(Suppl 12):3458S–3462S
Ray G, Batra S, Shukla NK et al (2000) Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat 59:163–170
Tas F, Hansel H, Belce A et al (2005) Oxidative stress in breast cancer. Med Oncol 22:11–15
Asal NR, Risser DR, Kadamani S et al (1990) Risk factors in renal cell carcinoma. I. Methodology, demographics, tobacco beverage use and obesity. Cancer Detect Prev 11:359–377
Gago-Dominguez M, Castelao JE, Yuan JM et al (2002) Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma. Cancer Causes Control 13:287–293
Sikka SC (2003) Role of oxidative stress response elements and antioxidants in prostate cancer pathobiology and chemoprevention—a mechanistic approach. Curr Med Chem 10:2679–2692
Aydin A, Arsova-Sarafinovska Z, Sayal A et al (2006) Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostate hyperplasia. Clin Biochem 39:176–179
Otamiri T, Sjodahl R (1989) Increased lipid peroxidation in malignant tissues of patients with colorectal cancer. Cancer 64:422–425
Oxdemirler G, Pabucçoglu H, Bulut T et al (1989) Increased lipoperoxide levels and antioxidant system in colorectal cancer. J Cancer Res Clin Oncol 124:555–559
Manoharan S, Kolanjiappan K, Suresh K et al (2005) Lipid peroxidation and antioxidants status in patients with oral squamous cell carcinoma. Ind J Med Res 2005; 122:529–534
Seril DN, Liao J, Yang GY et al (2003) Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis 34:353–362
Batcioglu K, Mehmet N, Ozturk IC et al (2006) Lipid peroxidation and antioxidant status in stomach cancer. Cancer Investig 24:18–21
Jaruga P, Zastawny TH, Skokowski J et al (1992) Oxidative DNA base damage and antioxidant enzyme activities in human lung cancer. FEBS Lett 341:59–64
Conklin KA (2004) Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther 3:294–300
Nicolson GL, Conklin KA (2006) Molecular replacement for cancer metabolic and mitochondrial dysfunction, fatigue and the adverse effects of cancer therapy. Cancer Genomics Proteomics 3:159–168
Conklin KA (2000) Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer 37:1–18
Betteridge DJ (2000) What is oxidative stress? Metabolism 49(Suppl 1):3–8
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenals, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Dianzani MU (1993) Lipid peroxidation and cancer. Crit Rev Oncol Hematol 15:125–147
Hauptlorenz S, Esterbauer H, Moll W et al (1985) Effects of the lipid peroxidation product 4-hydroxynonenal and related aldehydes on proliferation and viability of cultured Ehrlich ascites tumor cells. Biochem Pharmacol 34:3803–3809
Gonzalez MJ (1992) Lipid peroxidation and tumor growth: an inverse relationship. Med Hypotheses 38:106–110
Schackelford RE, Kaufmann WK, Paules RS (2000) Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med 28:1387–1404
Balin AK, Goodman DBP, Rasmussen H et al (1978) Oxygen-sensitive stages of the cell cycle of human diploid cells. J Cell Biol 78:390–400
Kurata S (2000) Selective activation of p38 MAPK cascade and mitotic arrest caused by low level oxidative stress. J Biol Chem 275:23413–23416
Wei Q, Frazier ML, Levin B (2000) DNA repair: a double edge sword. J Natl Cancer Inst 92:440–441
Fojo T (2001) Cancer, DNA repair mechanisms, and resistance to chemotherapy. J Natl Cancer Inst 93:1434–1436
Zhen W, Link CJ, O’Connor PM et al (1992) Increased gene-specific repair of cisplatin interstrand cross-links in cisplatin-resistant human ovarian cancer cell lines. Mol Cell Biol 12:3689–3698
Lee Y-J, Shacter E (1999) Oxidative stress inhibits apoptosis in human lymphoma cells. J Biol Chem 274:19792–19798
Hampton MB, Orrenius S (1997) Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett 414:552–556
Hampton MB, Fadeel B, Orrenius S (1998) Redox regulation of the caspases during apoptosis. Ann NY Acad Sci 854:328–335
Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29:323–333
Shacter E, Williams JA, Hinson RM et al (2000) Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood 96:307–313
Conklin KA (2005) Coenzyme Q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther 4:110–130
Lehninger AL (1951) Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide. J Biol Chem 190:345–359
Rasmussen UF, Rasmussen HN (1985) The NADH oxidase system (external) of muscle mitochondria and its role in the oxidation of cytoplasmic NADH. Biochem J 229:632–641
Nohl H (1987) Demonstration of the existence of an organo-specific NADH dehydrogenase in heart mitochondria. Eur J Biochem 169:585–591
Davies KJA, Doroshow JH (1986) Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261:3060–3067
Doroshow JH, Davies KJA (1986) Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 261:3068–3074
Nohl H (1988) Identification of the site of Adriamycin-activation in the heart cell. Biochem Pharmacol 37:2633–2637
Gille L, Nohl H (1997) Analyses of the molecular mechanism of Adriamycin-induced cardiotoxicity. Free Radic Biol Med 23:775–782
Eaton S, Skinner R, Hale JP et al (2000) Plasma coenzyme Q10 in children and adolescents undergoing doxorubicin therapy. Clin Chim Acta 302:1–9
Karlsson J, Folkers K, Astrom H et al (1986) Effect of Adriamycin on heart and skeletal muscle coenzyme Q10 (CoQ10) in man. In: Folkers K, Yamamura Y (eds) Biomedical and clinical aspects of coenzyme Q, vol 5, Elsevier/North-Holland Biomedical Press, Amsterdam, 241–245
Palmeira CM, Serrano J, Kuehl DW et al (1997) Preferential oxidation of cardiac mitochondrial DNA following acute intoxication with doxorubicin. Biochim Biophys Acta 1321:101–106
Serrano J, Palmeira CM, Kuehl DW et al (1999) Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim Biophys Acta 1411:201–205
Papadopoulou LC, Tsiftsoglou AS (1996) Effects of hemin on apoptosis, suppression of cytochrome C oxidase gene expression, and bone-marrow toxicity induced by doxorubicin. Biochem Pharmacol 52:713–722
Domae N, Sawada H, Matsuyama E et al (1981) Cardiomyopathy and other chronic toxic effects induced in rabbits by doxorubicin and possible prevention by coenzyme Q10. Cancer Treat Rep 65:79–91
Usui T, Ishikura H, Izumi Y et al (1982) Possible prevention from the progression of cardiotoxicity in Adriamycin-treated rabbits by coenzyme Q10. Toxicol Lett 12:75–82
Ghione M, Bertazzoli C (1977) CoQ and anthracycline associated cardiomyopathy. In: Folkers K, Yamamura Y (eds) Biomedical and clinical aspects of coenzyme Q, Elsevier/North-Holland Biomedical Press, Amsterdam, 183–199
Yamanaka N, Kato T, Nishida K et al (1980) Protective effect of coenzyme Q10 on Adriamycin toxicity and increase of antitumor effects of Adriamycin by coenzyme Q10. In: Yamamura Y, Folkers K, Ito Y (eds) Biomedical and clinical aspects of coenzyme Q, vol 2, Elsevier/North-Holland Biomedical Press, Amsterdam 213–224
Judy WV, Hall JH, Dugan W et al (1984) Coenzyme Q10 reduction of Adriamycin cardiotoxicity. In: Folkers K, Yamamura Y (eds) Biomedical and clinical aspects of coenzyme Q, vol 4, Elsevier/North-Holland Biomedical Press, Amsterdam, 231–241
Cortes EP, Gupta M, Chou C et al (1978) Adriamycin cardiotoxicity: early detection by systolic time interval and possible prevention by coenzyme Q10. Cancer Treat Rep 62:887–891
Iarussi D, Auricchio U, Agretto A et al (1994) Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med 15:S207–S212
Folkers K, Baker L, Richardson PC et al (1981) New progress on the biomedical and clinical research on coenzyme Q10. In: Folkers K, Yamamura Y (eds) Biomedical and clinical aspects of coenzyme Q, vol 3, Elsevier/North-Holland Biomedical Press, Amsterdam, 399–412
Okuma K, Ota K (1986) The effect of coenzyme Q10 on ECG changes induced by doxorubicin (Adriamycin). In: Folkers K, Yamamura Y (eds) Biomedical and clinical aspects of coenzyme Q, vol 5, Elsevier/North-Holland Biomedical Press, Amsterdam, 247–256
Takimoto M, Sakurai T, Kodama K et al (1982) Protective effect of CoQ 10 administration on cardial toxicity in FAC therapy. Gan To Kagaku Ryoho 9:116–121
Buckingham R, Fitt J, Sitzia J (1997) Patients’ experience of chemotherapy: side-effects of carboplatin in the treatment of carcinoma of the ovary. Eur J Cancer Care 6:59–71
Loke YK, Price D, Derry S et al (2006) Case reports of suspected adverse drug reactions—systematic literature survey of follow-up. Br Med J 232:335–339
Vogelzang N, Breitbart W, Cella D et al (1997) Patient caregiver and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. Semin Hematol 34(Suppl 2):4–12
Von Roenn JH, Paice JA (2005) Control of common, non-pain cancer symptoms. Semin Oncol 32:200–210
Nicolson GL (2005) Lipid replacement/antioxidant therapy as an adjunct supplement to reduce the adverse effects of cancer therapy and restore mitochondrial function. Pathol Oncol Res 11:139–144
Piper BF, Linsey AM, Dodd MJ (1987) Fatigue mechanism in cancer. Oncol Nurs Forum 14:17–23
McDonald E, David AS, Pelosi AJ et al (1993) Chronic fatigue in primary care attendees. Psychol Med 23:987–998
Nicolson GL (2003) Lipid replacement as an adjunct to therapy for chronic fatigue, anti-aging and restoration of mitochondrial function. J Am Nutraceut Assoc 6(3):22–28
Wei YH, Lee HC (2002) Oxidative stress, mitochondrial DNA mutation and impairment of antioxidant enzymes in aging. Exp Biol Med 227:671–682
Huang H, Manton KG (2004) The role of oxidative damage in mitochondria during aging: a review. Front Biosci 9:1100–1117
Logan AC, Wong C (2001) Chronic fatigue syndrome: oxidative stress and dietary modifications. Altern Med Rev 6:450–459
Manuel y Keenoy B, Moorkens G et al (2001) Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci 68:2037–2049
Richards RS, Roberts TK, McGregor NR et al (2000) Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep 5:35–41
Felle S, Mecocci P, Fano G et al (2000) Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radic Biol Med 29:1252–1259
Pall ML (2000) Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Med Hypotheses 54:115–125
Nicolson GL, Poste G, Ji T (1977) Dynamic aspects of cell membrane organization. Cell Surf Rev 3:1–73
Subczynski WK, Wisniewska A (2000) Physical properties of lipid bilayer membranes: relevance to membrane biological functions. Acta Biochim Pol 47:613–625
Radi R, Rodriguez M, Castro L et al (1994) Inhibition of mitochondrial electronic transport by peroxynitrite. Arch Biochem Biophys 308:89–95
Kanno T, Sato EE, Muranaka S et al (2004) Oxidative stress underlies the mechanism for Ca(2+)-induced permeability transition of mitochondria. Free Radic Res 38:27–35
Nicolson GL, Ellithrope R (2006) Lipid replacement and antioxidant nutritional therapy for restoring mitochondrial function and reducing fatigue in chronic fatigue syndrome and other fatiguing illnesses. J Chronic Fatigue Syndr 13(1):57–68
Agadjanyan M, Vasilevko V, Ghochikyan A et al (2003) Nutritional supplement (NTFactor) restores mitochondrial function and reduces moderately severe fatigue in aged subjects. J Chronic Fatigue Syndr 11(3):23–26
Ellithorpe RR, Settineri R, Nicolson GL (2003) Reduction of fatigue by use of a dietary supplement containing glycophospholipids. J Am Nutraceut Assoc 6(1):23–28
Seidman M, Khan MJ, Tang WX et al (2002) Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg 127:138–144
Colodny L, Lynch K, Farber C et al (2000) Results of a study to evaluate the use of Propax to reduce adverse effects of chemotherapy. J Am Nutraceut Assoc 2(1):17–25
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Garth L. Nicolson and Kenneth A. Conklin have no financial interest in any products discussed in this contribution.
Rights and permissions
About this article
Cite this article
Nicolson, G.L., Conklin, K.A. Reversing mitochondrial dysfunction, fatigue and the adverse effects of chemotherapy of metastatic disease by molecular replacement therapy. Clin Exp Metastasis 25, 161–169 (2008). https://doi.org/10.1007/s10585-007-9129-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-007-9129-z