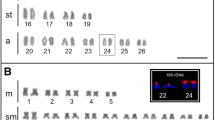
Abstract
To better understand genomic and chromosomal organization and evolutionary patterns of the U1 snRNA gene in cichlid fish, the gene was cytogenetically mapped and comparatively analyzed in 19 species belonging to several clades of the group. Moreover, the distribution and organization of U1 snRNA gene was analyzed in the Oreochromis niloticus genome. The results indicated high conservation of one chromosomal cluster of U1 snRNA in the African, Asian, and South American species, with few variations in the chromosomal position of the clusters in the South American species. The genomic analysis of U1 revealed a distinct scenario from that observed under the cytogenetic mapping. An enrichment of the U1 gene on linkage group (LG) 14 was observed that did not correspond to the same chromosome that harbors the U1 cluster identified by cytogenetic mapping. Moreover, it was revealed that the presence of several distinct transposable elements in the U1 gene flanking regions could be involved in the spreading of this sequence, but the generation of new, large snRNA clusters (detectable by fluorescent in situ hybridization, FISH) is apparently hampered. These results contribute to the understanding of multigene families' evolution and reinforce the utility of integrative analysis and the use of cytogenetic and bioinformatic methods to address the genomic and chromosomal evolutionary patterns of repeated DNAs among vertebrates. Moreover, the U1 gene represents a useful new chromosomal marker for cytogenetic studies.




Similar content being viewed by others
Abbreviations
- aa:
-
Amino acid
- BAC:
-
Bacterial artificial chromosome
- CLDN13:
-
Claudin 13 gene
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- FISH:
-
Fluorescence in situ hybridization
- FR:
-
Flanking region
- FRU:
-
Flanking region including the U1 gene
- LG:
-
Linkage group
- MYA:
-
Million years ago
- NCBI:
-
National Center for Biotechnology Information
- NTS:
-
Nontranscribed spacer
- ORF:
-
Open reading frame
- PCR:
-
Polymerase chain reaction
- snRNA:
-
Small nuclear RNA
- TE:
-
Transposable element
References
Barzotti R, Pelliccia F, Rocchi A (2003) Identification and characterization of U1 small nuclear RNA genes from two crustacean isopod species. Chromosome Res 11:365–373
Bertollo LAC, Takahashi CS, Moreira-Filho O (1978) Cytotaxonomic consideration on Hoplias lacerdae (Pisces, Erythrinidae). Brazilian J Genet 1:103–120
Bringmann P, Lührmann R (1986) Purification of the individual snRNPs U1, U2, U5 and U4/U6 from HeLa cells and characterization of their protein constituents. EMBO J 5:3509–3516
Brown DT, Morris GF, Chodchoy N, Sprecher C, Marzluff WF (1985) Structure of the sea urchin Ul RNA repeat. Nucleic Acid Res 13:537–556
Cabral-de-Mello DC, Cabrero J, Loópez-Leoón MD, Camacho JPM (2011a) Evolutionary dynamics of 5S rDNA location in acridid grasshoppers and its relationship with H3 histone gene and 45S rDNA location. Genetica 139:921–931
Cabral-de-Mello DC, Moura RC, Martins C (2011b) Cytogenetic mapping of rRNAs and histone H3 genes in 14 species of Dichotomius (Coleoptera, Scarabaeidae, Scarabaeinae) beetles. Cytogenet Genome Res 134:127–135
Cabrero J, Camacho JP (2008) Location and expression of ribosomal RNA genes in grasshoppers: abundance of silent and cryptic loci. Chromosome Res 16:595–607
Cabrero J, López-Leoón MD, Teruel M, Camacho JP (2009) Chromosome mapping of H3 and H4 histone gene clusters in 35 species of acridid grasshoppers. Chromosome Res 17:397–404
Cichlid Genome Consortium. http://cichlid.umd.edu/CGCindex.html. Accessed 20 August 2011
Drummond AJ, Ashton B, Cheung M, Heled J, Kearse M, Moir R, Stones-Havas S, Thierer T, Wilson A (2009) Geneious. v. 4.8.5. Available from http://www.geneious.com
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunesekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A (2010) The Pfam protein families database. Nucleic Acids Res 38:D211–D222
Genner MJ, Seehausen O, Lunt DH, Joyce DA, Shaw PW, Carvalho GR, Turner GF (2007) Age of cichlids: new dates for ancient lake fish radiations. Mol Biol Evol 24:1269–1282
Helfman GS, Collette BB, Facey DE (1997) The diversity of fishes. Blackwell Science, Malden
Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J (2005) Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res 110:462–467
Kapitonov VV, Jurka J (2006a) L1-55_XT family of frog non-LTR retrotransposons. Repbase Reports 6:628–628
Kapitonov VV, Jurka J (2006b) L1-56_XT family of frog non-LTR retrotransposons. Repbase Reports 6:629–629
Kapitonov VV, Jurka J (2006c) L1-53_XT family of frog non-LTR retrotransposons. Repbase Reports 6:626–626
Kapitonov VV, Jurka J (2007) hAT-1_NV—a family of autonomous DNA transposons from the starlet sea anemone genome. Repbase Reports 7:586–586
Katagiri T, Asakawa S, Minagawa S, Shimizu N, Hirono I, Aoki T (2001) Construction and characterization of BAC libraries for three fish species; rainbow trout, carp and tilapia. Anim Genetics 32:200–204
Katagiri T, Kidd C, Tomasino E, Davis JT, Wishon C, Stern JE, Carleton KL, Howe AE, Kocher TD (2005) A BAC-based physical map of the Nile tilapia genome. BMC Genomics 6:89
Kocher TD (2004) Adaptive evolution and explosive speciation: the cichlid fish model. Nature 5:288–298
Kohany O, Gentles AJ, Hankus L, Jurka J (2006) Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinforma 7:474
Kojima KK, Fujiwara H (2004) Cross-genome screening of novel sequence-specific non-LTR retrotransposons: various multicopy RNA genes and microsatellites are selected as targets. Mol Biol Evol 21:207–217
Kornfield I, Smith PF (2000) African cichlid fishes: model system for evolutionary biology. Ann Rev Ecol Syst 31:163–196
Lindgren V, Ares M, Weiner AM, Francke U (1985) Human genes for U2 small nuclear RNA map to a major adenovirus 12 modification site on chromosome 17. Nature 314:115–116
Lund E, Nesbitt MN (1988) The embryonic and adult mouse UI snRNA genes map to different chromosomal loci. Som Cell Mol Genet 14:143–148
Lund E, Bostock C, Robertson M, Christie S, Mitchen JL, Dahlberg JE (1983) Ul small nuclear RNA genes are located on human chromosome1 and are expressed in mouse–human hybrid cells. Mol Cell Biol 3:2211–2220
Manchado M, Zuasti E, Cross I, Merlo A, Infante C, Rebordinos L (2006) Molecular characterization and chromosomal mapping of the 5S rRNA gene in Solea senegalensis: a new linkage to the U1, U2, and U5 small nuclear RNA genes. Genome 49:79–86
Martins C, Galetti PM Jr (1999) Chromosome localization of 5S rRNA genes in Leporinus (Anostomidae, Characiformes). Chromosome Res 7:363–367
Martins C, Wasko AP, Oliveira C, Porto-Foresti F, Parise-Maltempi PP, Wright JM, Foresti F (2002) Dynamics of 5S rDNA in the tilapia (Oreochromis niloticus) genome: repeat units, inverted sequences, pseudogenes and chromosome loci. Cytogenet Genome Res 98:78–85
Marz M, Kirsten T, Stadler PF (2008) Evolution of spliceosomal snRNA genes in metazoan animals. J Mol Evol 7:594–607
Marzluff WF, Brown DT, Lobo S, Wang S (1983) Isolation and characterization of two mouse U1b small nuclear RNA genes. Nucleic Acids Res 11:6255–6270
Mattaj IW, Zeller R (1983) Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5′ and 3′ flanking homology with other RNA polymerase II transcribed genes. EMBO J 2:1883–1891
Mazzuchelli J, Yang F, Kocher TD, Martins C (2011) Comparative cytogenetic mapping of Sox2 and Sox14 in cichlid fishes and inferences on the genomic organization of both genes in vertebrates. Chromosome Res 19:657–667
Merlo MA, Cross I, Chairi H, Manchado M, Rebordinos L (2010) Analysis of three multigene families as useful tolls in species characterization of two closely-related species, Dicentrarchus labrax, Dicentrarchus punctatus and their hybrids. Genes Genet Syst 85:341–349
Nelson JS (2006) Fishes of the world. John Wiley and Sons, New York
Nilsen TW (2003) The spliceosome: the most complex macromolecular machine in the cell? BioEssays 25:1147–1149
Pelliccia F, Barzotti R, Bucciarelli E, Rocchi A (2001) 5S ribosomal and U1 small nuclear RNA genes: a new linkage type in the genome of a crustacean that has three different tandemly repeated units containing 5S ribosomal DNA sequences. Genome 44:331–335
Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci 83:2934–2938
Poletto AB, Ferreira IA, Cabral-de-Mello DC, Nakajima RT, Mazzuchelli J, Ribeiro HB, Venere PC, Nirchio M, Kocher TD, Martins C (2010a) Chromosome differentiation patterns during cichlid fish evolution. BMC Genet 11:50
Poletto AB, Ferreira IA, Martins C (2010b) The B chromosome of the cichlid fish Haplochromis obliquidens harbors 18S rRNA genes. BMC Genet 11:1
Pullin RSV (1991) Cichlids in aquaculture. In: Keenleyside MHA (ed) Cichlid fishes: behaviour, ecology and evolution. Chapman & Hall, New York, pp 280–309
Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV et al (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94
Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A et al (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453:1064–1071
Salzburger W, Meyer A (2004) The species flocks of East African cichlid fishes: recent advances in molecular phylogenetics and population genetics. Naturwissenschaften 91:277–290
Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Turner GF (2007) Adaptive radiation of cichlid fish. Curr Biol 17:827–831
Úbeda-Manzanaro M, Merlo MA, Palazón JL, Cross I, Sarasquete C, Rebordinos L (2010) Chromosomal mapping of the major and minor ribosomal genes, (GATA) n and U2 snRNA gene by double-colour FISH in species of the Batrachoididae family. Genetica 138:787–794
Valadkhan S (2005) snRNAs as the catalysts of pre-mRNA splicing. Curr Op Chem Biol 9:603–608
van Arsdell SW, Weiner AM (1984) Human genes for U2 small nuclear RNA are tandemly repeated. Mol Cell Biol 4:492–499
Watanabe-Nagasu NY, Itoh T, Tani T, Okano K, Koga N, Okada N, Ohshima Y (1983) Structural analysis of gene loci for rat U1 small nuclear RNA. Nucleic Acids Res 11:1791–1801
Wise JA, Weiner AM (1980) Dictyostelium small nuclear RNA D2 is homologous to rat nucleolar RNA U3 and is encoded by a dispersed multigene family. Cell 22:109–118
Xiong Y, Eickbush TH (1990) Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J 9:3353–3362
Acknowledgements
The authors are grateful to PC Venere for the fieldwork assistance, J Mazzuchelli for assistance with BAC probes hybridization, and to T Kocher for providing animal sample and BAC clones. This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) from Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fengtang Yang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material S1
a U1 snRNA gene copies identified in the O. niloticus genome version Tilapia_broad_v1 that were used as queries in the blast search against the NCBI nucleotide collection. The number of copies and their relative scaffolds are described. The numbers indicated by “|” represent the start and end positions in the scaffold. Bold, E value of blastn search. b The sequences of FRs used as queries in the blast search against the NCBI nucleotide collection. Up and down descriptions indicate that the sequences are upstream or downstream of the respective U1 copies. The numbers indicated by “|” represent the start and end positions in the scaffold. c The FRU sequences used as queries in Repbase analysis. The numbers indicated by “|” represent the start and end positions in the scaffold. Underlined bases represent the putative U1 copies; the arrow represents the direction of transcription. d The aa residues of translated ORFs with positive results after analysis in Repbase. These sequences were used as queries in Pfam analysis. The numbers indicated by “|” represents the start and end positions in the scaffold (DOC 106 kb)
Supplementary material S2
Excluded regions of data presented in Fig. 1 (DOC 41 kb)
Supplementary material S3
Blast results using the U1 copies of O. niloticus as queries against the NCBI nucleotide collection (DOC 195 kb)
Supplementary material S4
Comparative analysis of blast results of U1_6 with other U1 results (DOC 70 kb)
Supplementary material S5
Selection of representative blast results using the FRs of U1 snRNA gene as queries in the search against the NCBI nucleotide collection (DOC 26 kb)
Supplementary material S6
Schematic representation of the overlap of the U1 copies (blue arrows) and U1_LV (Lytechinus variegatus; purple arrows). Box, details of the sequences of Lytechinus variegatus (purple arrows) and T. nigroviridis genomes (orange arrow). The sequence of new_U1 identified in Repbase and alignment with other U1 copies are also shown. The numbers indicated by “|” represent the start and end positions in the scaffold (DOC 1195 kb)
Supplementary material S7
The aa residues of translated ORFs with positive results after analysis in Repbase followed by a table with a summary of the results obtained. The colors indicate the positions of the TEs in the sequences. The numbers indicated by “|” represent the start and end positions in the scaffold (DOC 47 kb)
Supplementary material S8
Alignment of predicted aa residues of O. niloticus like-TEs and several TEs (L1-55_XT, L1-56_XT, KenoFr1, KenoDr1, Tx1-13). The yellow arrows indicate the Pfam identified domains (DOC 367 kb)
Rights and permissions
About this article
Cite this article
Cabral-de-Mello, D.C., Valente, G.T., Nakajima, R.T. et al. Genomic organization and comparative chromosome mapping of the U1 snRNA gene in cichlid fish, with an emphasis in Oreochromis niloticus . Chromosome Res 20, 279–292 (2012). https://doi.org/10.1007/s10577-011-9271-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-011-9271-y