Abstract
Caveolin and caveolin containing rafts are involved in the signaling of growth factors in various cell types. Previous reports of our lab indicated a co-localization of caveolin and the high affinity nerve growth factor (NGF) receptor tyrosine kinase A (TrkA). Mutual effects have been observed among which a caveolin-1 knock-down resulted in an impairment of the NGF signaling cascade rather than in an increase of activity as expected from other growth factor reports. On the other hand, an over-expression of caveolin-1 impaired the NGF stimulated activity of p42/44 mitogen activated protein kinases (MAPK). In this study, we used a caveolin-1 scaffolding domain (CSD) peptide (cavtratin) of which an inhibitory effect on growth factor receptors was reported. Our data showed that cavtratin suppresses the NGF-induced phosphorylation of TrkA as well as the activation of MAPK in porcine oligodendrocytes significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurotrophins constitute a family of six members which include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3); they are endogenously produced signaling molecules and play an important role in a variety of cellular processes of neural and non-neural cells (Levi-Montalcini et al. 1996; Althaus and Richter-Landsberg 2000; Chao 2003; Vega et al. 2004). The initial step of the NGF signaling pathway via TrkA is tyrosine phosphorylation (Heumann 1994) followed by the activation of further downstream components due to the association of other proteins to tyrosine phosphorylated sites of TrkA by their SH2 or PTB domains (Pawson et al. 1993; Reichardt 2006).
Previous results had shown that oligodendrocytes (OL), which myelinate axons in the central nervous system, respond to NGF by inducing proliferation and enhancing process regeneration (Althaus et al. 1992, 1997). They contain caveolae-like flask-shape structures to which the high affinity NGF-receptor TrkA was partially localized (Schmitz et al. 2010). Caveolin serves as a structural component of caveolae. It contains a scaffolding domain (CSD) between amino acid 82 and 101 that facilitates an interaction with different signaling molecules. This could be shown by caveolin-1 mutants lacking a functional CSD (Vihanto et al. 2006). In different cell models it has already been shown that high levels of caveolin could inhibit signal transduction by virtue of the ability of CSD to interfere with the generation of signaling complexes (Engelman et al. 1998). Caveolin inhibits various signaling pathways such as epidermal growth factor (EGF) (Couet et al. 1997; Agelaki et al. 2009), Src family tyrosine kinases (Wanaski et al. 2003) or platelet derived growth factor receptor (Yamamoto et al. 1999) while the insulin receptor were kept in an activated state (Burgermeister et al. 2008).
In this study, we have investigated the influence of CSD on the TrkA/NGF signaling pathway in mature pig OL by using a cell-permeable synthetic peptide (cavtratin) that corresponds to the amino acids of the CSD. Our data indicate that cavtratin inhibits the phosphorylation of TrkA as well as the activity of downstream components of the NGF signaling pathway such as MAPK Erk1 and 2. These findings are relevant for a better understanding of the regulation of the oligodendroglial process formation after NGF and it may be of interest for the search for novel suppressor proteins against glial tumors.
Materials and Methods
Reagents
All chemicals of analytical grade were obtained either from Sigma–Aldrich (Taufkirchen, Germany) or Merck (Darmstadt, Germany); culture media and fetal calf serum were obtained from Biochrom (Berlin, Germany), Mezlocillin from Bayer (Leverkusen, Germany). ECL western blotting detection kit came from Amersham (Freiburg, Germany).
Peptides
The short N-terminal cytoplasmic region of caveolin-1 located between amino acid 82 and 101 (DGIWKASFTTFTVTKYWFYR), known as the caveolin scaffolding domain (CSD) was synthesized as a fusion peptide to the C-terminus of the Antennapedia internalization sequence (RQIKIWFQNRRMKWKK) and termed as cavtratin (Calbiochem/Merck, Darmstadt, Germany). This sequence of CSD exactly corresponds to the sequence of Sus scrofa caveolin-1 at this position (Wang et al. 2008). A scrambled peptide was used as a control (Calbiochem/Merck, Darmstadt, Germany). Before use, the powder were weighted and dissolved in DMSO to 10 mM and diluted to 1 mM with distilled water.
Cell Culture
OL were isolated from adult pig brain and cultivated on poly-d-lysine-coated Petri dishes or multiwell cultured plates by using an established protocol (Althaus et al. 1984; Bürgisser et al. 1988; Althaus and Klöppner 2006). Briefly, the white matter of mature pig (domestic) brains was dissected, minced, and sieved through nylon sieves of descending pore size; cells were collected after centrifugation of the cell tissue suspension onto a discontinuous Percoll gradient; cells were seeded and cultured on poly-d-lysine-coated Petri dishes or multiwall culture plates. The culturing protocol was as previously described (Althaus et al. 1991), except that the fetal calf serum in the culture medium was reduced to 1–5%; TEM and immunocytochemical criteria identified the cells as mature (GC+, MBP+, PLP+, MOC+) OL (Althaus and Klöppner 2006; Althaus et al. 1991; Althaus and Siepl 1997); A2B5+, GFAP+, or OX42+ cells were initially observed rarely if at all (Althaus and Siepl 1997); anti-MOSP IgM (Chemicon, Temecula, CA) diluted 1:1000 was routinely used to label OL (Dyer and Matthieu 1994) in this study.
Immunocytochemistry
For immunocytochemical staining of phospho-TrkA (P-TrkA) we used anti-P-TrkA IgG (Santa Cruz, Heidelberg, Germany) diluted 1:100 and as secondary antibody Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes/Invitrogen, Karlsruhe, Germany) diluted 1:1000. Moreover, polyclonal anti-caveolin IgG (Transduction Laboratories, Lexington, USA) diluted 1:1000 for western blot as well as monoclonal anti-beta actin IgG (Abcam, Cambridge, UK) diluted 1:10000 for western blot had been utilized.
After 8 DIV (days in vitro) under standard conditions, OL were pre-treated with cavtratin (25 and 40 μM). After 1 h incubation, NGF (100 ng/ml) was added into the cell medium. Under the same conditions OL were treated with a scrambled (non-sense) peptide.
For staining of P-TrkA OL were pre-treated with 1 mM sodium orthovanadate for 1 h, washed with PBS (containing a mixture of protease inhibitors: 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 1 μg/ml leupeptide). Afterwards, cells were fixed in methanol/acetic acid (9:1) and treated with 0.1% Triton X-100. The antibody incubation for the P-TrkA-staining took around 1 h at room temperature by smooth shaking.
Western Blotting
OL were pre-treated with 1 mM sodium orthovanadate for 1 h, washed with PBS (phosphate buffered saline) containing a mixture of inhibitors (1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 1 μg/ml leupeptide) and harvested by scraping on ice. The cells were lysed in 2% SDS, containing all inhibitors, for 30 min, denaturated in sample buffer (containing mercaptoethanol), and heated at 95°C for 2 min.
Protein samples of equivalent protein content (20 μg/ml; protein determination according to Neuhoff et al. 1979) were separated by 10–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli (1970) and transferred to polyvinylidene difluoride (PVDF) membranes (Amersham). PVDF membranes were blocked with 5% dried milk in PBS and 0.1% Tween-20 for 1 h at room temperature, and afterwards probed with the antibodies of interest overnight at 4°C. ECL anti-rabbit IgG from Amersham (diluted 1:1000) was utilized as secondary antibody. The bands were visualized by the enhanced chemiluminescence (ECL) detection system according to the manufacturer’s instructions.
Caveolin-1 Over-Expression
Previous results had shown that the level of caveolin-1 was up-regulated after stimulation of OL with NGF (100 ng/ml) for 48 h (Schmitz et al. 2010). To analyse the effect of caveolin-1 on the activity of MAPK (Erk and Erk2), 8 DIV OL were pre-incubated with NGF (100 ng/ml). After 48 h the medium was removed, cells were washed three times in PBS and incubated in fresh medium for 12 h under standard conditions. Afterwards the cells were incubated with NGF (100 ng/ml) for 4 h and the activity of MAPK (Erk1 and 2) were examined in an in-gel kinase assay in comparison to OL stimulated with NGF for 4 h without NGF pre-treatment.
In-Gel MAPK Assay
OL were exposed to NGF for the times indicated. The cell lysates were separated by SDS-PAGE using a gel in which 10% MBP (myelin basic protein) was incorporated. The renatured MAPK (Erk1 and Erk2) activity was detected following a modified protocol (Althaus et al., 1997) of Virdee and Tolkovsky (1995). The gel was incubated in an assay buffer for 60 min at 30°C allowing the transfer of radioactive phosphor to myelin basic protein. Not incorporated radioactivity was removed by washing the gel several times in 5% TCA (trichloroacetic acid) containing 1% (w/v) tetrasodium pyrophosphate. Afterwards the gel was dried overnight and subjected to autoradiography at −70°C, using an RX-Fuji X-ray film.
Statistics
The quantification of the signal intensities of all western blots was performed by Scion Image. Using the statistic program Graph Pad Prism 4 and the Student’s t test the ratios of different samples were tested for significance. All P values below 0.05 are considered as significant (*); under 0.01 as highly significant (**). The standard deviation (SD) was calculated to assess the variations between different samples under the same conditions and depicted as error bars. The error bars represent the SD of at least three independent attempts.
Results
Caveolin-1 Over-Expression After NGF Exposure Inhibited the Activity of MAPK
Previous publications had shown that NGF stimulates the oligodendroglial process formation by activating MAPK (Erk1 and 2) (Althaus et al. 1992, 1997). In this context, we observed an approximately twofold up-regulation of caveolin-1 expression in OL treated with NGF for 48 h compared to OL treated with NGF for 4 and 24 h or untreated OL (Fig. 1). We used this model to examine the MAPK activity in OL with an enhanced caveolin-1 expression in an in-gel kinase assay. OL were pre-incubated with NGF for 24 and 48 h. After 12 h under standard conditions, cells were incubated with NGF for 4 h and the activity of MAPK (Erk1 and 2) were examined in an in-gel kinase assay. Interestingly, OL pre-incubated with NGF for 48 h revealed a significant decrease of approximately 50% of MAPK activity (P < 0.05) after 4 h NGF exposure compared to OL pre-incubated with NGF for 24 h or treated with NGF for 4 h solely. Non-NGF treated control cells, showing a basal activity of MAPK, were set as 100% (Fig. 1).
Inhibition of MAPK activity after caveolin-1 up-regulation. OL, exposed to NGF for 48 h, revealed a significant up-regulation of caveolin-1 expression (P < 0.01) compared to untreated control cells and cells which were exposed to NGF for 4 or 24 h. An MAPK in-gel kinase assay showed that OL, pre-treated with NGF for 48 h, revealed a significant reduced activity of MAPK after 4 h NGF-incubation in comparison to cells, pre-incubated with NGF for 24 h or for 4 h solely. Untreated OL revealed a basal activity of MAPK. β-Actin was used as an internal control showing that equal amounts of protein had been loaded on the gel. For evaluation protein bands were quantified by Scion Image; control cells were set as 100%; data represent the average of three independent assays ± SD (standard deviation), calculated by Graph Pad Prism; when *P < 0.05 (student’s t test) the data were considered as significant
Effect of Cavtratin on the TrkA Phosphorylation Via NGF
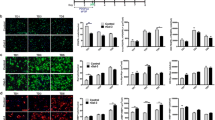
NGF induces a TrkA dimerization with subsequent tyrosine phosphorylation via its intrinsic tyrosine kinase activity (Reichardt 2006), a required step for the formation of oligodendroglial processes. Here, we investigated the effect of cavtratin on the phosphorylation of TrkA in pig OL before and after NGF stimulation. Without NGF addition, only few cells expressed phosphorylated TrkA (P-TrkA) as shown immunocytochemically and via western blotting (Figs. 2a3, 3a1).
Inhibition of the NGF-induced phosphorylation of TrkA via cavtratin. A few OL exhibit P-TrkA under standard conditions without NGF when labeled immunocytochemically with a rabbit polyclonal anti-phospho-TrkA IgG and an Alexa Fluor 488 conjugated second antibody (a3). Exposure to NGF (100 ng/ml) for 1 h, induced phosphorylation of TrkA in cells pre-treated with a scrambled peptide (a1), whereas pre-incubation with cavtratin (40 μM) for 1 h reduced TrkA phosphorylation significantly after NGF stimulation (P < 0.05) (a2). b The proportion of fluorescent cells versus non-fluorescent cells was determined. For evaluation the amount of fluorescent cells was calculated in % of total cells; data represent the average of three independent experiments ± SD, calculated by Graph Pad Prism; when *P < 0.05 (student’s t test) the data were considered as significant
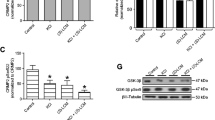
Detection and quantification of P-TrkA and MAPK (Erk1 and 2) after cavtratin treatment and NGF. a The phosphorylation of TrkA was determined by western blotting using anti-phospho-TrkA IgG. A treatment with NGF (100 ng/ml) for 1 h induced phosphorylation of TrkA in cells, pre-treated with a scrambled peptide (a2). Pre-incubation with cavtratin for 1 h reduced TrkA phosphorylation significantly (P < 0.05) (a3). Untreated cells showed a basal level of TrkA phosphorylation (a1). b Cavtratin treated OL were examined for Erk1/Erk2 activity in an in-gel kinase assay. After NGF exposure for 2 h (100 ng/ml), the MAPK activity of cavtratin pre-treated OL was significantly decreased (P < 0.01) (b5 cavtratin 25 μM and b6 cavtratin 40 μM), compared to cells, pre-incubated with a scrambled peptide (b3) and non-pretreated OL (b4). The MAPK activity of cells treated with cavtratin (40 μM) without NGF (b2) was comparable to that of untreated control cells (a1) which was set as 100% for the gray scale quantification. For gray scale evaluation protein bands were quantified by Scion Image; data represent the average of three independent assays ± SD, calculated by Graph Pad Prism; when *P < 0.05 (student’s t test) the data were considered as significant
Cells pre-incubated with the cell-permeable CSD peptide cavtratin (40 μM) revealed up to 40% reduced level of P-TrkA after exposure to NGF (Figs. 2a2, 3a3); whereas an increase of P-TrkA could be observed in OL treated with a scrambled peptide that are exposed to NGF for 1 h (Figs. 2a1, 3a2).
Influence of Cavtratin on the Activity of NGF Downstream Components Erk1 and Erk2 (MAPK)
Downstream components of the NGF signaling cascade such as the MAPK (Erk1 and Erk2), of which previous results had shown that oligodendroglial process regeneration depends on their activity (Althaus and Richter-Landsberg 2000).
OL, cultured for 8 DIV were pre-incubated with cavtratin and with a scrambled peptide for 1 h. After NGF treatment, OL pre-incubated with a scrambled peptide (Fig. 3b3) responded with an increased MAPK activity almost similarly to non-pre-incubated cells (Fig. 3b4). In contrast, OL pre-incubated with cavtratin responded to NGF with a significant decrease (~60%) of MAPK activity (P < 0.01) (Fig. 3b5 and 6). Without NGF stimulation, oligodendroglial MAPK activity remained at a basal level independent of cavtratin addition (Fig. 3b1, 2).
Discussion
Porcine OL contain caveolin containing rafts (CCRs) in their plasma membranes and express caveolin-1 (Schmitz et al. 2010; Althaus et al. 2008). Several studies on other cell types had provided evidence that caveolin may exert an inhibitory influence on signaling pathways (Couet et al. 1997), reduces inflammation (Bucci et al. 2000) and suppresses the progression of tumors (Gratton et al. 2003; Williams and Lisanti 2005). A short N-terminal cytoplasmic region of caveolin-1 located between amino acid 82 and 101, known as the CSD (Wanaski et al. 2003), appears to exert a nonselective, universal kinase inhibition (Couet et al. 1997; Liu et al. 2002) essential for the down regulation of signaling cascades (Razani and Lisanti 2001). Receptor tyrosine kinases such as EGFR (epidermal growth factor receptor) (Couet et al. 1997) or PDGFR (platelet derived growth factor receptor) (Yamamoto et al. 1999) are known targets which may be negatively regulated by CSD (Williams and Lisanti 2005); however, a CSD interaction with TrkA has not yet been shown. In case of pig OL caveolin-1 has been found in the oligodendroglial plasma membrane, where it was co-localized with signaling components of the NGF signaling cascade such as TrkA, P-TrkA, and MAPK (Althaus et al. 1992). Surprisingly, a knock-down of caveolin-1 resulted in a reduced rather than in an enhanced activity of these components. On the other hand, an increased up-regulation of caveolin-1 inhibited MAPK activity. These results seem to indicate that a balance of a caveolin level is important for regulating cellular signaling: a certain caveolin-1 level is required to keep the signaling of caveolin containing platforms in an optimal condition, whereas an increased caveolin-1 level counteracts signaling (Czarny et al. 1999). In this context we have asked the question could not only MAPK but also TrkA be inhibited by CSD directly. Our results revealed an inhibitory effect of cavtratin on the activity of p42/44 MAPK; a sustained reduced MAPK activity could impair the oligodendroglial process formation after NGF as previously shown (Schmitz et al. 2010). Other studies had already shown that the inhibitory activity of CSD is not limited to tyrosine kinases. It inhibits also serine/threonine kinases (Couet et al. 1997). These observations are consistent with previous in vitro and in vivo studies demonstrating that caveolin negatively regulates the p42/p44 MAPK by directly binding to catalytic domains of these kinases through the CSD (Engelman et al. 1998; Bianco et al. 2008). However, whether caveolin interacts with p42/44 MAPK and/or with a component upstream of MAPK has not yet been proven. Pig OL exposed to cavtratin revealed a decreased phosphorylation of TrkA after NGF treatment. Hence, TrkA appears to belong to the category of signal molecules which directly interact with the CSD (Bianco et al. 2008). These results may also be relevant for human OL, since a high homology exists between pig Trk/caveolin and human Trk/caveolin; in addition, TrkA is expressed in human OL (Althaus et al. 2001).
Furthermore, our findings that cavtratin reduced the activity of a glial growth factor signaling cascade, which promotes proliferation and process formation in OL, may be of interest not only in terms of myelination but it may also be relevant for interfering with glial tumors. Previous experiments on other cell types have suggested that a caveolin/cavtratin based treatment inhibits tumor growth (Gratton et al. 2003). In this respect the CSD of caveolin may also be considered as a novel therapeutic approach in glial pathology. The influence of cavtratin on the growth and differentiation of glioblastoma cells is currently under investigation.
Abbreviations
- CCR:
-
Caveolin containing raft
- CSD:
-
Caveolin scaffolding domain
- h:
-
Hours
- OL:
-
Oligodendrocytes
- MAPK:
-
Mitogen-activated protein kinases
- NGF:
-
Nerve growth factor
- TrkA:
-
Tyrosine kinase A
References
Agelaki S, Spiliotaki M, Markomanolaki H, Kallergi G, Mavroudis D, Georgoulias V, Stournaras C (2009) Caveolin-1 regulates EGFR-signaling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibition. Cancer Biol Ther 8:1470–1477
Althaus HH, Klöppner S (2006) Mature pig oligodendrocytes rapidly process human recombinant pro-nerve growth factor and do not undergo cell death. J Neurochem 98:506–517
Althaus HH, Richter-Landsberg C (2000) Glial cells as targets and producers of neurotrophins. Int Rev Cytol 197:203–277
Althaus HH, Siepl C (1997) Oligodendrocytes isolated from adult pig brain synthesise and release prostaglandins. Cell Tissue Res 287:135–141
Althaus HH, Montz H, Neuhoff V, Schwartz P (1984) Isolation and cultivation of mature oligodendroglial cells. Naturwissenschaften 71:309–315
Althaus HH, Schröter J, Spoerri P, Schwartz P, Klöppner S, Rohmann A, Neuhoff V (1991) Protein kinase C stimulation enhances the process formation of adult oligodendrocytes and induces proliferation. J Neurosci Res 29:481–489
Althaus HH, Klöppner S, Schmidt-Schultz T, Schwartz P (1992) Nerve growth factor induces proliferation and enhances fiber regeneration in oligodendrocytes isolated from adult pig brain. Neurosci Lett 135:219–223
Althaus HH, Hempel R, Klöppner S, Engel J, Schmidt-Schultz T, Kruska L, Heumann R (1997) Nerve growth factor signal transduction in mature pig oligodendrocytes. J Neurosci Res 50:729–742
Althaus HH, Mursch K, Klöppner S (2001) Differential response of mature TrkA/p75(NTR) expressing human and pig oligodendrocytes aging, does it matter? Microsc Res Tech 52:689–699
Althaus HH, Klöppner S, Klopfleisch S, Schmitz M (2008) Oligodendroglial cells and neurotrophins: a polyphonic cantata in major and minor. J Mol Neurosci 35:65–79
Bianco C, Strizzi L, Mancino M, Watanabe K, Gonzales M, Hamada S, Raafat A, Sahlah L, Chang C, Sotgia F, Normanno N, Lisanti M, Salomon DS (2008) Regulation of Cripto-1 signaling and biological activity by caveolin-1 in mammary epithelial cells. Am J Pathol 172:345–357
Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC (2000) In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6:1362–1367
Burgermeister E, Liscovitch M, Röcken C, Schmid RM, Ebert MP (2008) Caveats of caveolin-1 in cancer progression. Cancer Lett 268:187–201
Bürgisser P, Althaus HH, Rohmann A, Neuhoff V (1988) Lipid synthesis by oligodendrocytes from adult pig brain maintained in a long-term culture. Neurochem Int 13:111–118
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signaling pathways. Nat Rev Neurosci 4:299–309
Couet J, Sargiacomo M, Lisanti MP (1997) Interaction of a receptor tyrosine kinase EGF-R with caveolins, Caveolin binding negatively regulates tyrosine and serine/threonine kinase activity. J Biol Chem 272:30429–30438
Czarny M, Lavie Y, Fiucci G, Liscovitch M (1999) Localization of phospholipase D in detergent insoluble, caveolin-rich membrane domains. Modulation by caveolin-1 expression and caveolin-182-101. J Biol Chem 274:2717–2724
Dyer CA, Matthieu JM (1994) Antibodies to myelin/oligodendrocyte-specific protein and myelin/oligodendrocytes glycoprotein signal distinct changes in the organization of cultured oligodendroglial membrane sheets. J Neurochem 62:777–787
Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP (1998) Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett 428:205–211
Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, Iwakiri Y, Groszmann R, Claffey KP, Cheng YC, Sessa WC (2003) Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell 4:31–39
Heumann R (1994) Neurotrophin signalling. Curr Opin Neurobiol 4:668–679
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:685
Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A (1996) Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci 19:514–520
Liu P, Rudick M, Anderson RG (2002) Multiple functions of caveolin-1. J Biol Chem 277:41295–41298
Neuhoff V, Philipp K, Zimmer HG, Mesecke A (1979) A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Hoppe Seylers Z Physiol Chem 360:1657–1670
Pawson T, Olivier P, Rozakis-Adcock M, McGlade J, Henkemeyer M (1993) Proteins with SH2 and SH3 domains coupled receptor tyrosine kinases to intracellular signalling pathways. Philos Trans R Soc Lond B Biol Sci 340:279–285
Razani B, Lisanti MP (2001) Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. Am J Physiol Cell Physiol 281:1241–1250
Reichardt LF (2006) Neurotrophin-regulated signaling pathways. Philos Trans R Soc Lond B Biol Sci 361:1545–1564
Schmitz M, Klöppner S, Klopfleisch S, Möbius W, Schwartz P, Zerr I, Althaus HH (2010) Mutual effects of caveolin and nerve growth factor signaling in pig oligodendrocytes. J Neurosci Res 88:572–588
Vega JA, García-Suárez O, Germanà A (2004) Vertebrate thymus and the neurotrophin system. Int Rev Cytol 237:155–204
Vihanto MM, Vindis C, Djonov V, Cerretti DP, Huynh-Do U (2006) Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase. J Cell Sci 119:2299–2309
Virdee K, Tolkovsky AM (1995) Activation of p44 and p42 MAP kinases is not essential for the survival of rat sympathetic neurons. Eur J Neurosci 7:2159–2169
Wanaski SP, Ng BK, Glaser M (2003) Caveolin scaffolding region and the membrane binding region of SRC form lateral membrane domains. Biochemistry 42:42–56
Wang C, Mei Y, Li L, Mo D, Li J, Zhang H, Tian X, Chen Y (2008) Molecular characterization and expression analysis of caveolin-1 in pig tissue. Sci China C Life Sci 51:655–661
Williams TM, Lisanti MP (2005) Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol 288:494–506
Yamamoto M, Toya Y, Jensen RA, Ishikawa Y (1999) Caveolin is an inhibitor of platelet-derived growth factor receptor signaling. Exp Cell Res 247:380–388
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Schmitz, M., Zerr, I. & Althaus, H.H. Effect of Cavtratin, a Caveolin-1 Scaffolding Domain Peptide, on Oligodendroglial Signaling Cascades. Cell Mol Neurobiol 31, 991–997 (2011). https://doi.org/10.1007/s10571-011-9694-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9694-1