Abstract
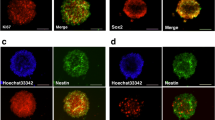
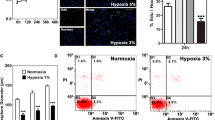
β-catenin, a protein that functions in both cell adhesion and Wnt signaling, plays a key role in mammalian neural development. To investigate the role of β-catenin in hyperbaric oxygen therapy (HBO)-induced neurogenesis after hypoxic ischemic brain damage (HIBD), we transfected β-catenin siRNA and negative control siRNA into neural stem cells (NSCs) after HIBD. We found that HBO promoted NSCs differentiate into neurons or oligodendrocytes, and inhibited NSCs differentiate into astrocytes; HIBD brain tissue extract conditioned cultures promoted NSCs differentiate into neurons; β-Catenin siRNA decreased the NSE-positive neurons and increased GFAP-positive astrocytes in the NSCs in vitro. Furthermore, the expression of Ngn1 protein and mRNA in NSCs was increased when HBO promoted NSCs differentiate into neurons after HIBD, and the expression of BMP-4 protein and mRNA was decreased when HBO depressed NSCs differentiate into astrocytes after HIBD. These results showed that β-catenin-mediated transcriptional activation functions in the decision of NSCs to proliferate neurogenesis during HBO-induced after HIBD, and suggested that HBO therapy promotes the proliferation of neural stem cells in vitro, an effect that may be correlated with β-catenin protein and HBO therapy could promote neurogenesis by β-catenin-induced activated Ngn1 gene and repress astrocytogenesis by β-catenin-induced down-regulated BMP-4 gene.




Similar content being viewed by others
References
Backman M, Machon O, Mygland L, van den Bout CJ, Zhong W, Taketo MM, Krauss S (2005) Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol 279:155–168
Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638–642
Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMa-hon AP, Sommer L, Boussadia O, Kemler R (2001) Inactivation of the (β)-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253–1264
Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M (2002) Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 22(4):275–279
Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297:365–369
Enrique MT, Marcela C, Nibaldo CI (2008) Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol 86:281–296
Fawke J, McIntyre J (2002) Recent advances in neonatology. Curr Opin Obstet Gynecol 14:153–158
Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z et al (2006) Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci 26:4359–4369
Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438
Goings GE, Sahni V, Szele FG (2004) Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res 996:213–226
Günther A, Küppers-Tiedt L, Schneider PM, Kunert I, Berrouschot J, Schneider D et al (2005) Reduced infarct volume and differential effects on glial cell activation after hyperbaric oxygen treatment in rat permanent focal cerebral ischemia. Eur J Neurosci 21:3189–3194
Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y (2004) The Wnt/β-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131(12):2791–2801
Ille F, Atanasoski S, Falk S, Ittner LM, Marki D, Buchmann-Moller S, Wurdak H, Suter U, Taketo MM, Sommer L (2007) Dev Biol 304:394–408
Lei Zhi-Nian, Zhang Lin-Mei, Sun Feng-Yan (2008) β-Catenin inhibits ischemia-induced striatal neurogenesis in adult rat brain following a transient middle cerebral artery occlusion. Neurosci Lett 435:108–112
Lin S-Y, Chen C-L, Wu Y-L, Yang Y-C, Hwu Y-M (2008) Ratio of Wnt3a to BMP4 doses is critical to their synergistic effects on proliferation of differentiating mouse embryonic stem cells. Cell Prolif 41:492–505
Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S (2003) Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience 122:129–143
Massari ME, Murre C (2000) Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20:429–440
Matchett GA, Martin RD, Zhang JH (2009) Hyperbaric oxygen therapy and cerebral ischemia: neuroprotective mechanisms. Neurol Res 31(3):114–121
Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483–1487
Rice JE, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131–141
Sanchez EC (2007) Hyperbaric oxygenation in peripheral nerve repair and regeneration. Neurol Res 29:184–198
Triulzi F, Parazzini C, Righini A (2006) Patterns of damage in the mature neonatal brain. Pediatr Radiol 36:608–620
Vehkamp R, Siebing DA, Heiland S, Schoenffeldt-Varas P, Veltkamp C, Schwaninger M et al (2005) Hyperbaric oxygen induces rapid protection against cerebral ischemia. Brain Res 1037:134–138
Vojtek AB, Taylor J, DeRuiter SL, Yu JY, Figueroa C, Kwok RP, Turner DL (2003) Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol 23:4417–4427
Wang XL, Yang YJ, Xie M et al (2007) Proliferation of neural stem cells correlates with Wnt-3 protein in hypoxic-ischemic neonate rats after hyperbaric oxygen therapy. Neuroreport 18(1629):1753–1756
Xie M, Yang YJ, Liu CT et al (2007) Differentiation of rat bone marrow stromal cells into neural cells induced by hypoxic-ischemic brain tissue extracts in neonate rats. J Cent South Univ (Med Sci) 32(4):557–562
Yanagisawa M, Takizawa T, Ochiaia W, Uemura A, Nakashima K, Taga T (2001) Fate alteration of neuroepithelial cells from neurogenesis to astrocytogenesis by bone morphogenetic proteins. Neurosci Res 41:391–396
Yang YJ, Wang XL, Yu XH et al (2008) Hyperbaric oxygen induces endogenous neural stem cells to proliferate and differentiate in hypoxic-ischemic brain damage in neonatal rats. Undersea Hyperb Med 35(2):113–129
Yu XH, Yang YJ, Wang X et al (2004) Hyperbaric oxygen therapy protects rats from hypoxic-ischemic brain damage. Chin J Contemp Pediatr 6(6):466–469
Yu XH, Yang YJ, Wang X, Wang QH, Xie M, Qi BX, Liu CT et al (2006) Effect of hyperbaric oxygenation on neural stem cells and myelin in neonatal rats with hypoxic-ischemic brain damage. Chin J Contemp Pediatr 8:33–37
Acknowledgments
This study was supported by a grant from the National Science Foundation of China (Grant No. 30672240 and Grant No. 30772341).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, XY., Yang, YJ., Xu, PR. et al. The Role of β-Catenin Signaling Pathway on Proliferation of Rats Neural Stem Cells After Hyperbaric Oxygen Therapy In Vitro. Cell Mol Neurobiol 31, 101–109 (2011). https://doi.org/10.1007/s10571-010-9559-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9559-z