Abstract
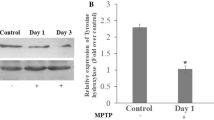
The authors investigated the protective effects of a novel astrocyte-modulating agent, arundic acid, in a 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine (MPTP) mouse model of Parkinson’s disease. Male mice received four intraperitoneal (i.p.) injections of MPTP (20 mg/kg) at 2 h intervals. The content of dopamine and its metabolites in the striatum was reduced markedly 7 days after MPTP treatment. The delayed treatment with arundic acid (30 mg/kg, i.p.) administered 3, 4, 5 and 6 days after MPTP treatment did not affect the depletion of dopamine and its metabolites in the striatum. Our immunohistochemical study with anti-tyrosine hydroxylase antibody, anti-neuronal nuclei antibody, anti-glial fibrillary acidic protein antibody, anti-S100β antibody and anti-nestin antibody showed that the delayed treatment with arundic acid had a protective effect against MPTP-induced neuronal damage in the striatum and the substantia nigra of mice. Furthermore, this agent ameliorated the severe reductions in number of isolectin reactive microglia in the striatum and the substantia nigra 7 days after MPTP treatment. These results demonstrate that the inhibition of S100β synthesis in astrocytes may be the major component of the beneficial effect of arundic acid. Thus, our present findings provide that the therapeutic strategies targeted to astrocytic modulation with arundic acid offers a great potential for restoring the functional capacity of the surviving dopaminergic neurons in individuals affected with Parkinson’s disease.





Similar content being viewed by others
References
Agid Y (1991) Parkinson’s disease: pathophysiology. Lancet 337:1321–1324
Araki T, Mikami T, Tanji H, Matsubara M, Imai Y, Mizugaki M, Itoyama Y (2001) Biochemical and immunohistological changes in the brain of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mouse. Eur J Pharm Sci 12:231–238
Bernheimer H, Birkmayer W, Hornykeiwicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Chen LW, Hu HJ, Liu HJ, Yung KKL, Chan YS (2004) Identification of BDNF in nestin-expressing astroglial cells in the neostriatum of MPTP-treated mice. Neuroscience 126:941–953
Chen LW, Zhang JP, Shum DKY, Chan YS (2006) Localization of nerve growth factor, neurotropin-3, and glial cell line-derived neurotrophic factor in nestin-expressing reactive astrocytes in the caudate-putamen of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/Bl mice. J Comp Neurol 497:898–909
Dahlstrand J, Lardelli M, Lendahl U (1995) Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res 84:109–129
Hasegawa E, Takeshige K, Oishi T, Murai Y, Minakami S (1990) 1-Methylphenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem Biophys Res Commun 170:1049–1055
Hayakawa N, Kato H, Araki T (2007) Age-related of astrocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev 128:673–677
Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM (2004) Tyrosine hydroxylase and dopamine transporter expression following 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res 76:539–550
Kato H, Araki T, Imai Y, Takahashi Y, Itoyama Y (2003) Protection of dopaminergic neurons with a novel astrocyte modulating agent (R)-(-)-2-propyloctanoic acid (ONO-2506) in an MPTP-mouse model of Parkinson’s disease. J Neurol Sci 208:9–15
Kato H, Kurosaki R, Oki C, Araki T (2004) Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res 1030:66–73
Katsumata S, Tateishi N, Kagamiishi Y, Shintaku K, Hayakawa T, Shimoda T, Shinagawa R, Akiyama T, Katsube N (1999) Inhibitory effect of ONO-2506 on GABAA receptor disappearance in cultured astrocytes and ischemic brain. Abstr Soc Neurosci 25:2108
Kernie SG, Erwin TM, Parada LF (2001) Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res 66:317–326
Kurosaki R, Akasak M, Michimata M, Matsubara M, Imai Y, Araki T (2003) Effects of Ca2+ antagonists on motor activity and the dopaminergic system in aged mice. Neurobiol Aging 24:315–319
Kurosaki R, Muramatsu Y, Kato H, Araki T (2004) Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson’s disease. Pharmacol Biochem Behav 78:143–153
Matsui T, Mori T, Tateishi N, Kagamiishi Y, Satoh S, Katsube N, Morikawa E, Morimoto T, Ikuta F, Asano T (2002) Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: Enhanced astrocytic synthesis of S-100β in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab 22:711–722
Mori A, Ohashi S, Nakai M, Moriizumi T, Mitsumoto Y (2005) Neuronal mechanisms underlying motor dysfunction as detected by the tail suspension test in MPTP-treated C57BL/6 mice. Neurosci Res 51:267–274
Muramatsu Y, Kurosaki R, Watanabe H, Michimata M, Matsubara M, Imai Y, Araki T (2003) The expression of S100 protein is related to neuronal damage in MPTP-treated mice. Glia 42:307–313
Nakai M, Mori A, Watanabe A, Mitsumoto Y (2003) 1-Methyl-4-phenylpyridinium (MPP+) decreases mitochondrial oxidation-reduction (REDOX) activity and membrane potential (Deltapsi(m)) in rat striatum. Exp Neurol 179:103–110
Ridet JL, Malhotra SK, Privat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20:507–577
Shimoda T, Tateishi N, Shintaku K, Yada N, Katagi J, Akiyama T, Maekawa H, Shinagawa R, Kondo K (1998) ONO-2506, a novel astrocyte modulating agent, suppresses the increase of COX-2 and iNOS mRNA expression in cultured astrocytes and ischemic brain. Abstr Soc Neurosci 24:984
Shinagawa R, Tateishi N, Shimoda T, Maekawa H, Yada N, Akiyama T, Matsuda S, Katsube N (1999) Modulating effects of ONO-2506 on astrocytic activation in cultured astrocytes from rat cerebrum. Abstr Soc Neurosci 25:2108
Shinagawa R, Tateishi N, Shimoda T, Shintaku K, Yada N, Honjyo K, Kagamiishi Y, Kondo K (1998) ONO-2506 ameliorates neurodegenation through inhibition of reduction of GLT-1 expression. Abstr Soc Neurosci 24:984
Sriram K, Pai KS, Boyd MR, Ravindranath V (1997) Evidence for generation of oxidative stress in brain by MPTP: in vitro and in vivo studies in mice. Brain Res 749:44–52
Tateishi N, Mori Y, Kagamiishi Y, Satoh S, Katsube N, Morikawa E, Morimoto T, Matsui T, Asano T (2002) Astrocytic activation and delayed infarct expansion following permanent focal ischemia in rats. Part II. Suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neuronal deficits. J Cereb Blood Flow Metab 22:723–734
Tipton KF, Singer TP (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem 61:1191–1206
Wu DC, Jakson-Lewis M, Vila M, Tieu K, Teismann C, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S (2002) Blocakade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci 22:1763–1771
Acknowledgements
This study was supported in part by the Grant-in-Aid for Scientific Research (12877163, 13671095 and 13670627) from the Ministry of Science and Education in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oki, C., Watanabe, Y., Yokoyama, H. et al. Delayed Treatment with Arundic Acid Reduces the MPTP-induced Neurotoxicity in Mice. Cell Mol Neurobiol 28, 417–430 (2008). https://doi.org/10.1007/s10571-007-9241-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9241-2