Abstract
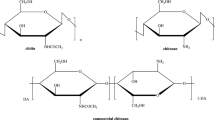
P-Aminosalicylhydrazide has successfully been applied as a cross linker for the epoxy chitosan Schiff’s base derivative. The aminosalicylhydrazide cross linked chitosan derivative was obtained by removing the benzaldehyde from its Schiff’s base derivative to regain the primary amine groups of chitosan. Two biocomposites based on this derivative filled with multi walled carbon nanotube (MWCNT) have been synthesized. Elemental analysis, FTIR spectroscopy, X-ray diffraction, scanning electron microscopy and transmission electron microscopy observations have been employed to prove the structure of the synthesized derivatives. The results showed that the investigated derivatives are more potent against the examined bacteria and fungi than the parent chitosan. They exhibited higher activity against Gram-positive bacteria than against Gram-negative bacteria. Some of them showed comparable or even greater activity than the used reference bactericides or fungicides. Thus, combination between chitosan and the used functionalized moieties as well as MWCNTs in one system has greatly improved the chitosan characteristics, may be considered as a route for achieving promising systems for antimicrobial agents which are taken as appropriate candidates in biomedical fields.








Similar content being viewed by others
References
Abd El-Ghany NA (2017) Antimicrobial activity of new carboxymethyl chitosan–carbon nanotube biocomposites and their swell ability in different pH media. J Carbohydr Chem 36:31–44
Ahmed S, Ikram S (2015) Chitosan & its derivatives: a review in recent innovations. IJPSR 6:14–30
Ahmed S, Ahmad M, Ikram S (2014) Chitosan: a natural antimicrobial agent: a review. J Appl Chem 3:493–503
Al-Jumaili A, Alancherry S, Bazaka K, Jacob MV (2017) Review on the antimicrobial properties of carbon nanostructures. Materials 10:1066–1091
Anan NA, Hassan SM, Saad EM, Butler IS, Mostafa SI (2011) Preparation, characterization and pH-metric measurements of 4-hydroxysalicylidene chitosan Schiff-base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Ru(III), Rh(III), Pd(II) and Au(III). Carbohydr Res 346:775–793
Annu Ahmed S, Ikram S (2017) Chitin and Chitosan: history, composition and properties, Chitosan. (Eds) S. Ahmed and S. Ikram, Scrivener & Wiley, New York, pp 3–24
Bhesaniya KD, Chanda SV, Baluja SH (2012) Epoxy aldehyde schiff bases: synthesis and antimicrobial study. Int J Pharm Res Scholars 1:6–10
Bhoi MN, Borad MA, Panchal NK, Patel HD (2015) 2-Aminobenzothiazole containing novel Schiff bases derivatives: search for new Antibacterial agents. ILCPA 53:106–113
Burkhanova ND, Yugai SM, Pulatova KP, Nikonovich GV, Milusheva RY, Voropaeva NL, Rashidova SS (2000) Structural investigations of chitin and its deacetylation products. Chem Nat Compd 36:352–355
Chien RC, Yen MT, Mau JL (2016) Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr Polym 138:259–264
Cuero RG, Osuji G, Washington A (1991) N-carboxymethylchitosan inhibition of aflatoxin production: role of zinc. Biotechnol Lett 13:441–444
Dorman H, Deans S (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88:308–316
Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, Abasi M, Hanifehpour Y, Joo SW (2014) Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett 9:393–405
El-Ghaouth A, Arul J, Grenier J, Asselin A (1992) Antifungal activity of chitosan on two post-harvest pathogens of strawberry fruits. Phytopathology 82:398–402
Entezari M, Tabatabaei ZG, Azarioun A, Sarabian S, Farahani GT (2015) In vitro evaluation of the antibacterial activity of modified multi-walled carbon nanotubes with phenolic extracts. J Biomater Tissue Eng 2:17–23
Eweis M, Elkholy SS, Elsabee MZ (2006) Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int J Biol Macromol 38:1–8
Fattouch S, Caboni P, Coroneo V, Tuberoso C, Angioni A, Dessi S, Marzouki N, Cabras P (2007) Antimicrobial activity of Tunisian Quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agric Food Chem 55(3):963–969
Feng QL, Wu J, Chen GO, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mat Res 52:662–668
Gemma S, Kukreja G, Fattorusso C, Persico M, Romano MP, Altarelli M, Savini L, Campiani G, Fattorusso E, Basilico N, Taramelli D, Yardley V, Butini S (2006) Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg Med Chem Lett 16:5384–5388
Gyawali R, Ibrahim SA (2014) Natural products as antimicrobial agents. Food Control 46:412–429
Hadwiger LA, Kendra DF, Fristensky BW, Wagoner W, Muzzarelli RAA, Jeuniaux C, Gooday CW (eds) (1986) Chitin in nature and technology. Plenum Press, New York, pp 209–214
Henderson DK (2006) Managing methicillin-resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms. Am J Infect Control 34:S46–S54
Jarrahpour A, Shirvani P, Sharghi H, Aberi M, Sinou V, Latour C, Brunel JM (2015) Synthesis of novel mono- and bis-Schiff bases of morpholine derivatives and the investigation of their antimalarial and antiproliferative activities. Med Chem Res 24:4105–4112
Kenawy E, Abdel-Hay FI, Mohy Eldin MS, Tamer TM, Ibrahim EMA (2015) Novel aminated chitosan-aromatic aldehydes Schiff bases: synthesis, characterization and bio-evaluation. IJAR 3:563–572
Kerfahi D, Tripathi BM, Singh D, Kim H, Lee S, Lee J, Adams JM (2015) Effects of functionalized and raw multi-walled carbon nanotubes on soil bacterial community composition. PLoS ONE 10(3):e0123042
Kong M, Chen XG, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63
Liang J, Chen Y, Ren X, Wu R, Barnes K, Worley SD, Broughton RM, Cho U, Kocer H, Huang TS (2007) Fabric treated with antimicrobial N-halamine epoxides. Ind Eng Chem Res 46:6425–6429
Malik S, Nema B (2016) Antimicrobial activities of schiff bases: a review. IJTAS 8:28–30
Mazimba O, Majinda RR, Masesane IB (2011) Synthesis and antibacterial activities of cyclodimers of styrene oxides. Bull Chem Soc Ethiop 25:299–304
Mohamed NA, Abd El-Ghany NA (2012) Synthesis and antimicrobial activity of some novel terephthaloyl thiourea cross-linked carboxymethyl chitosan hydrogels. Cellulose 19:1879–1891
Mohamed NA, Abd El-Ghany NA (2017) Synthesis, characterization and antimicrobial activity of chitosan hydrazide derivative. Int J Polym Mater Polym Biomater 66:410–415
Mohamed NA, Abd El-Ghany NA (2018) Novel aminohydrazide cross-linked chitosan filled with multi-walled carbon nanotubes as antimicrobial agents. Int J Biol Macromol 115:651–662
Mohamed NA, Al-mehbad NY (2013) Novel terephthaloyl thiourea cross-linked chitosan hydrogels as antibacterial and antifungal agents. Int J Biol Macromol 57:111–117
Mohamed NA, Sabaa MW, El-Ghandour AH, Abdel-Aziz MM, Abdel-Gawad OF (2013) Quaternized N-substituted carboxymethyl chitosan derivatives as antimicrobial agents. Int J Biol Macromol 60:156–164
Mohamed NA, Mohamed RR, Seoudi RS (2014) Synthesis and characterization of some novel antimicrobial thiosemicarbazone O-carboxymethyl chitosan derivatives. Int J Biol Macromol 63:163–169
Mohamed NA, Abd El-Ghany NA, Fahmy MM (2016) Novel antimicrobial superporous cross-linked chitosan/pyromellitimide benzoyl thiourea hydrogels. Int J Biol Macromol 82:589–598
Mohamed NA, Abd El-Ghany NA, Fahmy MM, Khalaf-Alla PA (2018) Novel polymaleimide containing dibenzoyl hydrazine pendant group as chelating agent for antimicrobial activity. Int J Polym Mater Polym Biomater 67:68–77
Mohan M, Gupta NS, Kumar A, Kumar M (1987) Synthesis, characterization and antitumour activity of iron(II) and iron(IlI) complexes of 3- and 5-substituted salicylaldehyde benzoyl hydrazones. Inorg Chim Acta I35:167–177
Munoz-B A, Fernandez-G M (2012) Polymeric materials with antimicrobial activity. Prog Polym Sci 37:281–339
Musumeci T, Puglisi G (2013) 10-Antimicrobial agents. In: Pignatello, R. (eds) Drug–Biomembrane Interaction Studies. Woodhead Publishing, Sawston, Cambridge, pp 305–333
Nawar N, Hosny NM (2000) Synthesis, spectral and antimicrobial activity studies of o-aminoacetophenone o-hydroxybenzoylhydrazone complexes. Trans Metal Chem 25:1–8
Nazzaro F, Caliendo G, Arnesi G, Veronesi A, Sarzi P, Fratianni F (2009) Comparative content of some bioactive compounds in two varieties of Capsicum annuum L. sweet pepper and evaluation of their antimicrobial and mutagenic activities. J Food Biochem 33(6):852–868
Oliveira CS, Airoldi C (2014) Pyridine derivative covalently bonded on chitosan pendant chains for textile dye removal. Carbohydr Polym 102:38–46
Pickart L, Goodwin WH, Burgua W, Murphy TB, Johnson DK (1983) Inhibition of the growth of cultured cells and an implanted fibrosarcoma by aroylhydrazone analogs of the Gly-His-Lys-Cu (II) complex. Biochem Pharmacol 32:3868–3871
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Ragavendran J, Sriram D, Patel S, Reddy I, Bharathwajan N, Stables J, Yogeeswari P (2007) Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur J Med Chem 42:146–151
Rahman A, Choudhary M I, Thompson W J (2001) Bioassay techniques for drug development. Harwood Academic Publishers, The Netherlands, vol 16, p 2024
Rathore HS, Mittal S, Kumar S (2000) Synthesis, characterization and antifungal activities of 3d- transition metal complexes of 1-acetylpiperazinyldithiocarbamate, M(acpdtc)2. Pestic Res J 12:103–107
Silverstein RM, Bassler GC, Morril TC (2000) Spectrometric identification of organic compounds, 6th edn. Wiley, New York
Todeschini AR, Miranda AL, Silva CM, Parrini SC (1998) Synthesis and evaluation of analgesic, antiinflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives Barreiro. Eur J Med Chem 33:189–199
Venkatesan J, Jayakumar R, Mohandas A, Bhatnagar I, Se-K Kim (2014) Antimicrobial activity of chitosan-carbon nanotube hydrogels. Materials 7:3946–3955
Vicini P, Zani F, Cozzini P, Doytchinova I (2002) Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 37:553–564
Xia D, Wu X, Shi J, Yang Q, Zhang Y (2011) Phenolic compounds from the edible seeds extract of Chinese Mei (Prunus mume Sieb. et Zucc) and their antimicrobial activity. LWT Food Sci Technol 44(1):347–349
Yogeshkumar NG, Atul SG, Adhikrao VY (2013) Chitosan and its applications: a review of literature. IJRPBS 4:312–331
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamed, N.A., Abd El-Ghany, N.A. Synthesis, characterization and antimicrobial activity of novel aminosalicylhydrazide cross linked chitosan modified with multi-walled carbon nanotubes. Cellulose 26, 1141–1156 (2019). https://doi.org/10.1007/s10570-018-2096-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2096-5