Abstract
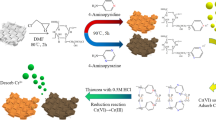
Cotton fibers containing different amounts of aldehyde and carboxyl groups were prepared by varying TEMPO oxidation conditions and then employed as carriers for trypsin immobilization. Depending on the functional group type present in the oxidized fiber, trypsin was immobilized through the ionic interaction or together with the Schiff’s base formation. The TEMPO oxidation caused changes in the chemical and physical properties of the modified cotton, which were evaluated by determining the aldehyde and carboxyl group content, sorption and electrokinetic properties, and surface morphology of fibers. The trypsin activity was assayed with N-α-benzoyl-dl-arginine p-nitroanilide hydrochloride, and the protein content was determined by the Bradford method. The impact of oxidized fiber functional groups on the amount, activity and stability of the immobilized trypsin was investigated. The cotton fibers oxidized under the most severe conditions (4.84 mmol/g NaClO, 180 min) and having the highest functional groups content (0.79 mmol/g) exhibited the maximum amount of immobilized trypsin of about 6.5 mg/g. However, the immobilized activity and the stability were much higher for the fibers containing both carboxyl and aldehyde groups compared to the corresponding fibers containing only carboxyl groups.







Similar content being viewed by others
References
ASTM D 2402-78 (1978) Standard test method for water retention of fibers (centrifuge method)
Bajerová M, Krejčová K, Rabišková M, Gajdziok J, Masteiková R (2009) Oxycellulose: significant characteristics in relation to its pharmaceutical and medical applications. Adv Polym Technol 28:199–208
Bolivar JM, Wilson L, Ferrarotti SA, Fernandez-Lafuente R, Guisan JM, Mateo C (2007) Evaluation of different immobilization strategies to prepare an industrial biocatalyst of formate dehydrogenase from Candida boidinii. Enzyme Microb Technol 40:540–546
Bragd PL, van Bekkum H, Besemer AC (2004) TEMPO-mediated oxidation of polysaccharides: survey of methods and applications. Top Catal 27:49–66
Cao L (2005) Carrier-bound immobilized enzymes: principles, applications and design. Wiley, Weinheim
Costa SA, Azevedo HS, Reis RL (2005) Enzyme immobilization in biodegradable polymers for biomedical applications. In: Reis RL, Román JS (eds) Biodegradable systems in tissue engineering and regenerative medicine. CRC Press, Boca Raton, pp 358–386
Dai L, Dai H, Yuan Y, Sun X, Zhu Z (2011) Effect of tempo oxidation system on kinetic constants of cotton fibers. BioResources 6:2619–2631
Di Risio S, Yan N (2009) Adsorption and inactivation behavior of horseradish peroxidase on cellulosic fiber surfaces. J Colloid Interface Sci 338:410–419
Fakin D, Golob V, Stana-Kleinschek K, Marechal AML (2006) Sorption properties of flax fibers depending on pretreatment processes and their environmental impact. Text Res J 76:448–454
Herbold CW, Miller JH, Coheen SC (1999) Cytochrome c unfolding on an anionic surface. J Chromatogr A 863:137–146
Horii F, Hirai A, Kitamaru R (1983) Solid-state 13C-NMR study of conformations of oligosaccharides and cellulose–conformation of CH2OH groups about the exo-cyclic C–C bond. Polym Bull 10:357–361
Karra-Châabouni M, Bouaziz I, Boufi S, Botelho do Rego AM, Gargouri Y (2008) Physical immobilization of Rhizopus oryzae lipase onto cellulose substrate: activity and stability studies. Colloids Surf B Biointerfaces 66:168–177
Kollár P, Suchy P, Muselik J, Bajerová M, Havelka P, Sopuch T (2008) Hemostatic effects of oxidized cellulose. Ceska Slov Farm 57:11–16
Kotel’nikova NE, Mikhailova SA, Vlasova EN (2007) Immobilization of proteolytic enzymes trypsin and α-chymotrypsin to cellulose matrix. Rus J Appl Chem 80:322–329
Kovalenko GA (1998) Immobilized proteolytic enzymes for external use. Pharm Chem J 32:213–216
Kruger NJ (2002) The Bradford method for protein quantitation. In: Walker JM (ed) The protein protocols handbook. Humana Press, Totowa, pp 15–21
Kumar V, Yang T (2002) HNO3/H3PO4–NaNO2 mediated oxidation of cellulose—preparation and characterization of bioabsorbable oxidized celluloses in high yields and with different levels of oxidation. Carbohydr Polym 48:403–412
Mendoza L, Mamo G, Flores N, Gimenez A, Hatti-Kaul R (2011) Laccase mediator system for activation of agarose gel: application for immobilization of proteins. J Mol Catal B Enzym 68:270–274
Nikolic T, Kostic M, Praskalo J, Pejic B, Petronijevic Z, Skundric P (2010) Sodium periodate oxidized cotton yarn as carrier for immobilization of trypsin. Carbohydr Polym 82:976–981
Nikolic T, Milanovic J, Kramar A, Petronijevic Z, Lj Milenkovic, Kostic M (2014) Preparation of cellulosic fibers with biological activity by immobilization of trypsin on periodate oxidized viscose fibers. Cellulose 21:1369–1380
Nouaimi M, Möschel K, Bisswanger H (2001) Immobilization of trypsin on polyester fleece via different spacers. Enzyme Microb Technol 29:567–574
O’Sullivan AC (1997) Cellulose: the structure slowly unravels. Cellulose 4:173–207
Ohta K, Makinen KK, Loesche WJ (1986) Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun 53:213–220
Parks EJ, Hebert RL (1972) Thermal analysis of ion-exchange reaction products of wood pulps with calcium and aluminum cations. Tappi J 55:1510–1514
Praskalo JZ (2010) Obtaining of special properties cellulose fibers by chemical modification. Dissertation, University of Belgrade
Praskalo J, Kostic M, Potthast A, Popov G, Pejic B, Skundric P (2009) Sorption properties of TEMPO-oxidized natural and man-made cellulose fibers. Carbohydr Polym 77:791–798
Reischl M, Stana-Kleinschek K, Ribitsch V (2006) Electrokinetic investigations of oriented cellulose polymers. Macromol Symp 244:31–47
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 5:1983–1989
Saito T, Okita Y, Nge TT, Sugiyama J, Isogai A (2006) TEMPO-mediated oxidation of native cellulose: microscopic analysis of fibrous fractions in the oxidized products. Carbohydr Polym 65:435–440
Secundo F (2013) Conformational changes of enzymes upon immobilization. Chem Soc Rev 42:6250–6261
Seyednejad H, Imani M, Jamieson T, Seifalian AM (2008) Topical haemostatic agents. Br J Surg 95:1197–1225
Spangler D, Rothenburger S, Nguyen K, Jampani H, Weiss S, Bhende S (2004) In vitro antimicrobial activity of oxidized regenerated cellulose against antibiotic-resistant microorganisms. Surg Infect 4:255–262
Stana-Kleinschek K, Ribitsch V (1998) Electrokinetic properties of processed cellulose fibers. Colloids Surf A Physicochem Eng Asp 140:127–138
Stana-Kleinschek K, Kreze T, Ribitsch V, Strnad S (2001) Reactivity and electrokinetical properties of different types of regenerated cellulose fibres. Colloids Surf A Physicochem Eng Asp 195:275–284
Villalonga R, Villalonga ML, Gómez L (2000) Preparation and functional properties of trypsin modified by carboxymethylcellulose. J Mol Catal B Enzym 10:483–490
Xi F, Wu J, Jia Z, Lin X (2005) Preparation and characterization of trypsin immobilized on silica gel supported macroporous chitosan bead. Process Biochem 40:2833–2840
Yackel EC, Kenyon WO (1942) The oxidation of cellulose by nitrogen dioxide. J Am Chem Soc 64:121–127
Yudanova TN, Reshetov IV (2006) Drug synthesis methods and manufacturing technology. Modern wound dressings: making and properties. II. Wound dressings containing immobilized proteolytic enzymes (a review). Pharm Chem J 40:430–434
Yui Y, Tanaka C, Isogai A (2013) Functionalization of cotton fabrics by TEMPO-mediated oxidation. Sen’i Gakkaishi 69:222–228
Zhukovskii VA (2005) Current status and prospects for development and production of biologically active fibre material for medical applications. Fibre Chem 37:352–355
Acknowledgment
This study has been supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project OI 172029). The authors also thank Andjelika Bjelajac (Faculty of Technology and Metallurgy, University of Belgrade) for obtaining the SEM images.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nikolic, T., Korica, M., Milanovic, J. et al. TEMPO-oxidized cotton as a substrate for trypsin immobilization: impact of functional groups on proteolytic activity and stability. Cellulose 24, 1863–1875 (2017). https://doi.org/10.1007/s10570-017-1221-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1221-1