Abstract
Using two cationic methacrylate polymers: poly([2-(methacryloyloxy)ethyl] trimethyl ammonium iodide) (PDMQ) and poly[(stearyl methacrylate)-stat-([2-(methacryloyloxy)ethyl] trimethyl ammonium iodide)] (PSMA13Q), we modified microfibrillated cellulose (MFC) water suspensions. The aim was to affect the flocculation and rheological behavior of the MFC suspension. PDMQ is a strongly cationic polymer while PSMA13Q, also a cationic polymer, contains hydrophobic segments. We studied the MFC/polymer suspension rheological properties with a rotational rheometer in oscillatory and flow measurements. To observe structural changes in suspensions at different shear rates, we measured flow curves with transparent outer geometry and photographed the sample with a digital camera. The oscillatory measurements showed that a small amount of the cationic PDMQ in the MFC suspension strengthened the gel, whereas a small amount of amphiphilic PSMA13Q weakened it. Increased amounts of either polymer increased the gel strength. PSMA13Q also changed the rheological character of the MFC suspension turning it more fluid-like. When we photographed the flow curve measurement, we saw a clear change in the floc structure. This floc structure rupture coincided with a transient region in the flow curve.
Similar content being viewed by others
Introduction
Microfibrillated cellulose (MFC) is a nanomaterial with unique, interesting properties. It can be used as a thickener and dispersing agent (Turbak et al. 1983), reinforcing material in nanocomposites (Nakagaito and Yano 2004; Nakagaito and Yano 2005), or for strengthening paper products (Taipale et al. 2010). MFC is commonly made from wood pulp (Turbak et al. 1983; Herrick et al. 1983; Pääkkö et al. 2007; Saito et al. 2006), though other resources, like sugar beet pulp (Dinand et al. 1996; Lowys et al. 2001; Goussé et al. 2004; Agoda-Tandjawa et al. 2010) or potato pulp (Dufresne et al. 2000) exist. Originally, Turbak et al. (1983) and Herrick et al. (1983) manufactured MFC solely by high shearing forces, but today, mild enzymatic hydrolysis (Pääkkö et al. 2007) or oxidation (Saito et al. 2006) are occasionally used prior to mechanical treatment. Resulting fibrils have a high aspect ratio and diameter varying between 5 and 100 nm depending on the raw materials (Saito et al. 2006; Iwamoto et al. 2008) and the manufacturing procedure (Saito et al. 2007; Abe et al. 2007).
MFC forms an entangled and disordered network consisting of fibrils obtained by mechanical disintegration of wood fibers (Pääkkö et al. 2007). Due to the strong interfibrillar forces, MFC forms a gel in water suspension even at a low (0.125%) concentration (Pääkkö et al. 2007). The network structure results in high viscosity at low shear rates and a pronounced shear thinning behavior with increasing shear rates (Pääkkö et al. 2007; Dinand et al. 1996; Agoda-Tandjawa et al. 2010; Iotti et al. 2011). This makes MFC an excellent thickener and dispersing agent (Turbak et al. 1983). At rest, the MFC network consists of sintered flocs that separate under flow. The separation of flocs and the following structural changes have been widely studied for pulp suspension (Wågberg and Nordqvist 1999; Björkman 2003a; Björkman 2003b; Chen et al. 2002; Beghello 1998) but not yet for MFC which has higher aspect ratio than macroscopic cellulose fibers. In pulp suspensions, the prevailing floc structure and size are dependent on shear rate and flow type. The floc structure in turn affects to the rheological properties of the suspension (Björkman 2006; Swerin 1998). Furthermore, flocculation can be affected by adding polyelectrolytes (Wågberg and Nordqvist 1999) or salts (Beghello 1998; Ono et al. 2004) to fiber suspensions or adjusting pH (Beghello 1998). Rheological properties like viscosity at different shear rates and flocculation are essential information for both the processing of MFC and its applications.
Different polymers have been mixed with MFC/water suspension to affect the rheological properties of the suspensions (Lowys et al. 2001; Vesterinen et al. 2010a; Vesterinen and Seppälä 2008) or to the aggregation of the fibers (Myllytie et al. 2009), but the results have not been combined. Our group (Vesterinen et al. 2010a) studied the addition of carboxymethylcellulose, cationic polyacrylamide, and cationic starch to MFC suspensions. Carboxymethylcellulose made the suspension less gel-like and reduced the shear thinning, whereas cationic polyacrylamide and starch strengthened the gel and preserved its shear thinning behavior. Using an optical microscope, Myllytie et al. (2009) observed that carboxymethylcellulose dispersed MFC fibers, whereas high molecular weight, low charged cationic starch, and especially polyacrylamide aggregated the fibers.
Applications of MFC may need modification of MFC’s properties. For example, the use of MFC as a paper strength agent requires cationic polyelectrolyte as a fixative (Taipale et al. 2010). On the other hand, the very hydrophilic nature of MFC can hinder its use as reinforcing material in less hydrophilic matrices. We focus on the effect of the cationic polymethacrylates on the MFC/water suspension rheology. Two kinds of polymethacrylates have been studied: cationic poly([2-(methacryloyloxy)ethyl] trimethyl ammonium iodide) (PDMQ) and amphiphilic, cationic statistical copolymer of 2-(methacryloyloxy)ethyl trimethyl ammonium iodide and stearyl methacrylate (PSMA13Q). PDMQ and its copolymers adsorb on negative charged silica surfaces or paper sheets by electrostatic adsorption from water solution (Nurmi et al. 2009; Österberg 2000). Thus, PSMA13Q is expected to adsorb on MFC fibers and to introduce hydrophobic segments on the fibers. PSMA13Q forms associated structures in water solution and therefore it is assumed to affect the fiber–fiber interactions in MFC suspensions, thus changing the fiber flocculation behavior. These modifications could generate MFC easier to store and handle, making it readily available for practical applications needing charged or hydrophobic surface.
Experimental
Materials
Microfibrillated cellulose was obtained from UPM-Kymmene Corporation. It was prepared from never dried birch pulp by mechanical disintegration. First, the pulp was passed once through a Voith refiner (Voith AG, Heidenheim, Germany) and then seven times through Fluidizer M7115 (Microfluidics Corp., Newton, MA, USA). The first pass was through a chamber with a diameter of 500 μm at pressure 300 bar, the second through two serially connected Z-shaped chambers with diameters of 500 and 200 μm, and the rest through chambers with diameters of 500 and 100 μm at pressure 1,850 bar. The final solid content of the water suspension was 1.6 wt%, and the fibers contained about 25 wt% xylan. Corresponding material has earlier been shown to consist of fibrils that have diameters visibly less than 50 nm, but with thicker fibril aggregates (Littunen et al. 2011).
The polymers used were cationized poly([2-(methacryloyloxy)ethyl] trimethyl ammonium iodide) (PDMQ) and cationized statistical copolymer of 2-(methacryloyloxy)ethyl trimethyl ammonium iodide (METAI) and stearyl methacrylate (SMA) (PSMA13Q). Figure 1 illustrates the structure of the monomers. The synthesis of prepolymer was performed in bulk phase with radical polymerization and cationic groups were obtained by postmodification. A detailed description of the synthesis and polymer characterization, including rheological characterization of the polymers, is reported elsewhere (Vesterinen et al. 2010b). Table 1 presents the molar masses and zero shear rate viscosities of the polymers in 1% water solution.
Preparation of suspensions
The polymers were dissolved in distilled water to desired concentrations. PSMA13Q needed heating to 60 °C. The MFC suspension was diluted from 1.6 wt% to 1 wt%, and then the polymer solution and MFC suspension were mixed. The concentration of MFC was 0.5 wt% and polymer concentrations were 0.25, 0.5, 1, 2, or 4 wt%. The suspensions were mixed with a magnetic stirrer for 45 min.
Rheological measurements
Viscoelastic properties of the suspensions were measured with a stress controlled rotational rheometer AR G2 (TA Instruments) using bob and cup geometry (ISO 3219/DIN 53 019) with a peltier element temperature control. The gap between the concentric cylinders was 0.5 mm. Drying was prevented with a cover during the measurement. Before measuring, the samples were allowed to rest 15 min in the geometry. Each sample was measured with a strain sweep, frequency sweep, and steady state flow. In the strain sweep, the strain was from 0.1 to 1,000% at an angular frequency 1.0 rad/s. After this, the samples were allowed to rest for 30 min. Next, a frequency sweep was done at 0.2% strain, in the linear viscoelastic region, varying angular frequency from 300 to 0.05 rad/s. Before the steady state flow, the sample was kept for 5 min at rest. Steady state flow was measured by raising the shear rate from 0.002 to 1,000 1/s. Percentage tolerance for the steady state was 5% and maximum point time 1 min, if the steady state was not reached. Measurements were carried out at 23 °C.
Apparent yield stresses were determined from the flow curve at shear rates 20–502.4 1/s using the Herschel-Bulkley calculation (Herschel and Bulkley 1926; Barnes 1999) which has been used earlier for pulp fiber suspensions (Derakhshandeh et al. 2010) and MFC suspensions (Lowys et al. 2001). The Herschel-Bulkley equation is presented below:
where τ is shear stress, τ0 is apparent yield stress, k consistency, \( \dot{\gamma } \) shear rate, and n is fluid behavior index.
Some suspensions were measured with a transparent outer geometry in order to photograph the changes in the suspension structure during the steady state flow measurements. The measuring conditions were the same as above, but the standard metal cup of the rheometer was replaced with a tailor-made polymethylmethacrylate (PMMA) cup of the same dimensions. The cup was located into a transparent water container to prevent reflections. The photographs were taken with Nikon D90 (Nikon Corporation, Japan) camera controlled by NKRemote software (Breeze Systems Limited, UK).
Results and discussion
Effect of polymers to gel-character and gel strength
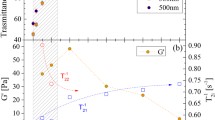
The frequency sweeps illustrate the gel-character of MFC suspensions, and how the two polyelectrolytes affect storage and loss modulus (G′ and G″) of the MFC suspension. Figure 2 presents the frequency sweeps of pure MFC and MFC suspension with addition of 0.5 or 4% of PDMQ or PSMA13Q. Pure MFC suspension (0.5 wt%) had a higher storage modulus than loss modulus, and the moduli were roughly independent of the angular frequency (Fig. 2a) indicating that the suspension was gel-like. MFC’s gel-like nature is well established in the literature (Pääkkö et al. 2007; Dinand et al. 1996; Lowys et al. 2001; Agoda-Tandjawa et al. 2010). An addition of cationic PDMQ to the MFC suspension did not affect the slopes of the moduli (Fig. 2b and c), whereas the amphiphilic, cationic PSMA13Q increased especially the slope of the loss modulus. The effect was already visible at a 0.5% addition of PSMA13Q, but was more pronounced when 4% amphiphilic polymer was added (Fig. 2d, e). This means that the gel-character of the suspension remained unchanged with the addition of the cationic polymer, whereas the suspension became more fluid-like with amphiphilic polymer addition.
Even at the lower concentration (0.5 wt% PDMQ), the cationic PDMQ increased the storage and loss modulus (Fig. 2b). This is in agreement with our earlier results (Vesterinen et al. 2010a), which showed that cationic polyelectrolytes strengthened MFC suspension while maintaining gel-like behavior. By contrast, the amphiphilic cationic PSMA13Q decreased the storage and loss modulus at a concentration of 0.5 wt% (Fig. 2d) but increased them at a concentration of 4 wt% PSMA13Q (Fig. 2e). Finally, PDMQ resulted in a higher storage modulus than PSMA13Q when the concentration of the polyelectrolyte was 4 wt%.
Figure 3 shows the storage modulus measured in the strain sweep. All the suspensions contained 0.5 wt% MFC and different amounts of PDMQ or PSMA13Q. The same trend observed in the frequency sweeps is seen here: the cationic PDMQ increased the storage modulus of MFC at both concentrations, while 0.5 wt% PSMA13Q decreased the storage modulus but 4% PSMA13Q increased it.
The storage modulus of the suspension measured in strain sweep increased with the addition of 0.5 wt% PDMQ to twice of the storage modulus of pure MFC suspension. The 0.5 wt% PDMQ is such a small amount that it hardly affects the properties of suspending medium. Thus, the change in the storage modulus of the gel is assumed to be due to a change on the MFC fiber surfaces and interactions between them. MFC has a slightly negative surface (Pääkkö et al. 2007), and as a cationic polymer, PDMQ adsorbs on negative surfaces and it is supposed to have also nonelectrostatic interactions with cellulose surface (Österberg 2000). The conformation of the adsorbed polymer is most likely flat, since PDMQ’s charge density is high and it is in extended conformation in water (Nurmi et al. 2009; Vesterinen et al. 2010b). Therefore, it is assumed that PDMQ had a good coverage on the fiber surface and it changed the surface of the fibers positive (illustrated in Fig. 4a). Increasing surface charge has been observed to hinder aggregation of cellulose microcrystals (Araki et al. 1998). 4 wt% PDMQ increased the storage modulus even more. The higher concentration may have increased the adsorption of PDMQ on the fibers producing a more positive charged surface (illustrated in Fig. 4b) and simultaneously the entangled fibrils still held the network together, leading to a strong gel. In addition to the effect on the electrostatic forces, polyelectrolytes can form bridges between cellulose surfaces (Salmi et al. 2007), thus affecting the fiber–fiber interactions. However, the bridging was not expected to play a significant role in these suspensions, since the molecular weights of the polymers were low.
A schematic illustration of the presumed effects of the two polyelectrolytes in two concentrations on the MFC fibers (thinner line = cationic segments, bolder line = hydrophobic segments). a 0.5 wt% of PDMQ adsorbed on the MFC fibers inducing slightly positive fiber surface. b 4 wt% of PDMQ converted the surface clearly positive. c 0.5 wt% amphiphilic PSMA13Q adsorbed in aggregated conformation on the fibers, thus the surface coverage of the polymer is lower. d 4 wt% PSMA13Q covered the fibers with a positive charge, but also introduced hydrophobic segments on the fibers
In contrast to PDMQ, a small amount of PSMA13Q (0.5 wt%) decreased the storage modulus compared to pure MFC. PSMA13Q probably adsorbed on the fibers as a more aggregated, less flat conformation, because the stearyl segments are not soluble in water and have no adhesion to the fibers (Nurmi et al. 2009). PSMA13Q also introduced hydrophobic segments on the surface. Figure 4c presents a schematic illustration of this. One reason for the lower storage modulus could be that the surface coverage of the positive charge was lower, which resulted in a partial screening of the negative charges. This allowed the negative and positive fiber surfaces to attach to each other which can induce aggregation of the fibers. Alternatively, the tendency of PSMA13Q to form intra and inter molecular associations (Vesterinen et al. 2010b) through the hydrophobic stearyl segments may have enhanced the aggregation. With a notably higher concentration of 4 wt%, PSMA13Q increased the storage modulus compared to pure MFC, but the increase was lower with PSMA13Q than with PDMQ. 4% PSMA13Q was probably able to turn the surface of the fibers positive, similarly to PDMQ, though hydrophobic segments can still cause some aggregation of the fibers (illustrated in Fig. 4d). However, since MFC is a very heterogeneous material, further research would be required to reveal the mechanism that causes the changes in the gel strength with PDMQ or PSMA13Q addition.
Effect of polymer to flocculation and flow curve
The MFC suspension structure changes when it is sheared with increasing shear rate (Björkman 2003a). At rest, the fibrils form a flocculated network that ruptures first to chain-like floc formations at low shear rates and then to individual flocs at higher shear rates. We expected this behavior to change further with polyelectrolyte addition. To follow the changes, we measured flow curves for all the suspensions with a common metal bob and cup geometry and with a transparent PMMA cup connected to a digital camera for selected suspensions. We were thus able to link the MFC structure to the rheological data. The results with normal metal measuring geometry are presented first. Then, we connect the details in the flow curve to the photographs taken during the measurement with transparent geometry.
Figures 5 and 6 present the steady state flow curves of the suspensions. MFC showed strongly shear-thinning behavior, as reported in the literature (Pääkkö et al. 2007; Dinand et al. 1996; Lowys et al. 2001; Agoda-Tandjawa et al. 2010; Iotti et al. 2011). A Newtonian plateau was not visible at measured low shear rates, and zero shear rate viscosity cannot be defined for the suspension. Between the regions of exponential shear thinning, an irregular region is seen at shear rates 2–20 1/s. Several studies show a similar behavior: see e.g. Agoda-Tandjawa et al. (2010), Iotti et al. (2011) and Chen et al. (2002). Earlier, it has been connected to structure changes in pulp suspensions (Chen et al. 2002).
Flow curve as a function of shear rate for MFC suspension at a concentration of 0.5 wt% and with addition of 0–4 wt% PSMA13Q. The letters a–f refers to the photos in Fig. 7
Adding the cationic PDMQ to the MFC suspension increased the flow viscosity, while the flow curve shape remained roughly the same (Fig. 5). The transition between the exponential shear thinning regions, however, started at lower shear rates with the addition of PDMQ. An increased PDMQ concentration retained the position of the transition region at the same shear rate. Accordingly, PDMQ did not affect the fundamental rheological nature of the MFC suspension. The positive polymer strengthened the gel at the small strain (Fig. 3), but when the suspension starts to flow, PDMQ does not significantly affect the viscosity after the initial increase.
The addition of amphiphilic PSMA13Q also increased the viscosity of the MFC suspension, but unlike PDMQ, it drastically altered the flow curve shape (Fig. 6). The position of the transition region moved towards lower shear rates with increasing PSMA13Q concentration. The amphiphilic character of PSMA13Q increased the flow viscosity, which could be due to the tendency of the polymer to intermolecular association. PSMA13Q was earlier found to form inter and intra molecular associations in water solution at concentrations higher than 0.8 wt% (Vesterinen et al. 2010b).
The photos in the Fig. 7 indicate that the transition region observed in the flow curve is related to the changes in the floc structure of the suspensions. Figure 7a–c show the structure of the pure MFC suspension at various shear rates, and Fig. 7d–f show MFC suspensions with the addition of 0.5 wt% of PSMA13Q at various shear rates. Figure 7a and d are taken at the first linear region of the flow curve (see Fig. 6), and the suspension appears relatively homogeneous. This is a consequence of the fiber network retaining an intact constitution from the previous shear interval.
Photographs of MFC suspensions without and with PSMA13Q during the flow curve measurement. Pure MFC suspension (0.5 wt%) at shear rate a 0.08 1/s, uniform network of small flocs; b 5 1/s, break-up of the network; and c 32 1/s, flow of individual, small flocs. MFC suspension (0.5 wt%) with PSMA13Q (0.5 wt%) at shear rate d 0.08 1/s, uniform network of small flocs; e 1.3 1/s, break-up of the network (lower shear rate than above); and f 32 1/s, flow of individual, small flocs
Figure 7b is of the flow curve transition region, and it shows how the structure of the flocculated MFC network breaks up. This demonstrates that the transition between the two linear regions in the flow curve arises from a visible structural change in the suspension. The same change is seen in Fig. 7e which is of the transition region of the flow curve for MFC suspensions with PSMA13Q addition. The transition region occurred at lower shear rates with the addition of PSMA13Q, and is again related to the change in the floc structure. The amphiphilic polymer induced the earlier break up of the even floc network, probably caused by the hydrophobic segments on the fibers. In Fig. 7c and f, taken at the second linear region of the flow curves where the fibrils flow in individual small flocs, the structure again appears quite uniformed.
Apparent yield stress
For flow property comparison of different suspensions, we used the Herschel-Bulkley equation to obtain apparent yield stress. The Herschel-Bulkley equation does not describe the transition in the middle of the flow curve, and therefore we only analyzed the shear rates higher than the transition, those between 20 and 502.4 1/s. In that region, the suspension is certainly at the state that it flows. The validity of the yield stress concept has been under debate (Barnes 1999; Derakhshandeh et al. 2010; Masalova et al. 2008). We agree that the obtained value is dependent on determination method and therefore comparable rather than absolute material characteristic. It may be a useful measure, however, given that it sensitively reflects changes resulting from added polyelectrolytes.
Figure 8 illustrates the fitting of the Herschel-Bulkley equation for three different suspensions and indicates that it is a good mathematical description of the suspensions within the plotted shear rates. A similar fitting was gained with all concentrations.
Figure 9 shows the apparent yield stress by Herschel-Bulkley equation as a function of polyelectrolyte concentration. Even at low concentrations, the cationic PDMQ raised the yield stress. At PDMQ concentrations of 0.5–2 wt%, the yield stress was roughly constant, and notably higher at a polymer concentration of 4 wt%. This indicates that the cationic polyelectrolyte increased the stress needed to break the coherent network in the gel. Higher concentrations probably increased the adsorbed amount of the cationic polyelectrolyte on the fiber, leading to a stronger gel. In contrast, amphiphilic PSMA13Q decreased the yield stress of MFC suspension at low concentrations of polymer (0.25–1 wt%). Hydrophobic parts on the fibers may have induced the break of the network at a lower stress. At higher concentrations, compared to pure MFC, amphiphilic PSMA13Q strengthened the suspension probably due to increased interfibrillar repulsion by the positive charge of the METAI segments on the fibers. The results are in good accordance with the gel strength measured in the strain sweep (Fig. 3).
Conclusions
We studied the effects of two cationic polyelectrolytes on the rheological behavior of MFC suspension at rest in oscillatory measurements and under shear flow conditions in flow measurements. In addition, we connected the rheological characterization under flow to the structure of the suspension by image analysis. At rest, the cationic methacrylate polymer PDMQ already strengthened the gel structure at small concentrations, possibly due to the increased interfibrillar repulsion by the positive charge. In contrast, a small amount of amphiphilic, cationic methacrylate polymer PSMA13Q weakened the fiber suspension, and as much as 4% PSMA13Q was needed to increase the gel strength at rest. The reason for this can be the hydrophobic segments of the PSMA13Q which formed an aggregated, less flat conformation of the polymer. In addition, PSMA13Q changed the rheological nature of the MFC suspension and made the suspension more fluid-like, whereas cationic PDMQ did not affect to the gel-like behavior of MFC.
Under flow conditions, MFC suspensions showed strongly shear-thinning behavior. The flow curves showed a transition region between two exponentially shear thinning regions, and this was studied closer with transparent outer geometry in the rheometer. We showed that this transition originates from the structural change in the suspension’s floc structure. Both polyelectrolytes moved this transition region towards lower shear rates, but the effect was more pronounced with PSMA13Q. In addition, PSMA13Q spread the transition to a wider shear rate range than PDMQ and the reference MFC suspension.
To obtain apparent yield stress for the suspensions, we fitted the flow curve data to the Herschel-Bulkley equation. The equation described the results well, and the yield stresses were in good accordance with gel strengths measured in dynamic measurements. It showed that the cationic PDMQ increased the yield stress at all studied concentrations but the amphiphilic PSMA13Q decreased the yield stress at lower concentrations. An increase in yield stress was finally seen with addition of 2 or 4% of PSMA13Q.
Adding a low molecular mass, cationic PDMQ offers a simple way to turn the surface of MFC fibers cationic without drastically changing the rheological characteristics of MFC suspension, hence making it useful in applications like paper making. Alternatively, adding an amphiphilic copolymer provides the possibility of modifying the rheological character of the suspensions to more fluid-like and changing the flocculation behavior. Our results suggest that tailor-made copolymers can be used to modify MFC and make it suitable for various applications.
References
Abe K, Iwamoto S, Yano H (2007) Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 8:3276–3278
Agoda-Tandjawa G, Durand S, Berot S, Blassel C, Gaillard C, Garnier C, Doublier J-L (2010) Rheological characterization of microfibrillated cellulose suspensions after freezing. Carbohydr Polym 80:677–686
Araki J, Wada M, Kuga S, Okano T (1998) Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf Physicochem Eng Aspects 142:75–82
Barnes HA (1999) The yield stress—a review or ‘παντα ρε ι’—everything flows? J Non Newtonian Fluid Mech 81:133–178
Beghello L (1998) Some factors that influence fiber flocculation. Nord Pulp Pap Res J 13:274–279
Björkman U (2003a) Break-up of suspended fibre networks. Nord Pulp Pap Res J 18:32–37
Björkman U (2003b) Stress generation and transmission in suspended fibre networks. Nord Pulp Pap Res J 18:38–43
Björkman U (2006) Floc dynamics in flowing fibre suspensions. Nord Pulp Pap Res J 20:247–252
Chen B, Tatsumi D, Matsumoto T (2002) Floc structure and flow properties of pulp fiber suspensions. J Soc Rheol Japan 30:19–25
Derakhshandeh B, Hatzikiriakos SG, Bennington CPJ (2010) The apparent yield stress of pulp fiber suspensions. J Rheol 54:1137–1154
Dinand E, Chanzy H, Vignon M (1996) Parenchymal cell cellulose from sugar beet pulp: preparation and properties. Cellulose 3:183–188
Dufresne A, Dupeyre D, Vignon MR (2000) Cellulose microfibrils from potato tuber cells: processing and characterization of starch–cellulose microfibril composites. J Appl Polym Sci 76:2080–2092
Goussé C, Chanzy H, Cerrada ML, Fleury E (2004) Surface silylation of cellulose microfibrils: preparation and rheological properties. Polymer 45:1569–1575
Herrick FW, Casebier RL, Hamilton JK, Sandberg KR (1983) Microfibrillated cellulose: morphology and accessibility. J Appl Polym Sci Appl Polym Symp 37:797–813
Herschel WH, Bulkley R (1926) Konsistenzmessungen von Gummi-Benzollösungen. Kolloid Z 39:291–300
Iotti M, Gregersen Ø, Moe S, Lenes M (2011) Rheological studies of microfibrillar cellulose water dispersions. J Polym Environ 19:137–145
Iwamoto S, Abe K, Yano H (2008) The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics. Biomacromolecules 9:1022–1026
Littunen K, Hippi U, Johansson L, Österberg M, Tammelin T, Laine J, Seppälä J (2011) Free radical graft copolymerization of nanofibrillated cellulose with acrylic monomers. Carbohydr Polym 84:1039–1047
Lowys MP, Desbrières J, Rinaudo M (2001) Rheological characterization of cellulosic microfibril suspensions. Role of polymeric additives. Food Hydrocoll 15:25–32
Masalova I, Malkin AY, Foudazi R (2008) Yield stress of emulsions and suspensions as measured in steady shearing and in oscillations. Appl Rheol 18:44790-1–44790-8
Myllytie P, Holappa S, Paltakari J, Laine J (2009) Effect of polymers on aggregation of cellulose fibrils and its implication on strength development in wet paper web. Nord Pulp Pap Res J 24:125–134
Nakagaito AN, Yano H (2004) The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl Phys A 78:547–552
Nakagaito AN, Yano H (2005) Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl Phys A 80:155–159
Nurmi L, Holappa S, Nykänen A, Laine J, Ruokolainen J, Seppälä J (2009) Ultra-thin films of cationic amphiphilic poly(2-(dimethylamino)ethyl methacrylate) based block copolymers as surface wettability modifiers. Polymer 50:5250–5261
Ono H, Shimaya Y, Sato K, Hongo T (2004) 1H spin–spin relaxation time of water and rheological properties of cellulose nanofiber dispersion, transparent cellulose hydrogel (TCG). Polym J 36:684–694
Österberg M (2000) the effect of a cationic polyelectrolyte on the forces between two cellulose surfaces and between one cellulose and one mineral surface. J Colloid Interface Sci 229:620–627
Pääkkö M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindström T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941
Saito T, Nishiyama Y, Putaux J, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7:1687–1691
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8:2485–2491
Salmi J, Österberg M, Stenius P, Laine J (2007) Surface forces between cellulose surfaces in cationic polyelectrolyte solutions: the effect of polymer molecular weight and charge density. Nord Pulp Pap Res J 22:249–257
Swerin A (1998) Rheological properties of cellulosic fibre suspensions flocculated by cationic polyacrylamides. Colloids Surf Physicochem Eng Aspects 133:279–294
Taipale T, Österberg M, Nykänen A, Ruokolainen J, Laine J (2010) Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength. Cellulose 17:1005–1020
Turbak AF, Snyder FW, Sanberg KR (1983) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci Appl Polym Symp 37:815–827
Vesterinen A, Seppälä J (2008) Rheological study of microfibrillar cellulose and dynamic mechanical analysis of paper sheet. Annu Trans Nord Rheol Soc 16:259–262
Vesterinen A, Myllytie P, Laine J, Seppälä J (2010a) The effect of water-soluble polymers on rheology of microfibrillar cellulose suspension and dynamic mechanical properties of paper sheet. J Appl Polym Sci 116:2990–2997
Vesterinen A, Rich J, Seppälä J (2010b) Synthesis and solution rheology of poly[(stearyl methacrylate)-stat-([2-(methacryloyloxy)ethyl] trimethyl ammonium iodide)]. J Colloid Interface Sci 351:478–484
Wågberg L, Nordqvist T (1999) Detection of polymer induced flocculation of cellulosic fibres by image analysis. Nord Pulp Pap Res J 14:247–255
Acknowledgments
This work was a part of the Nanosellu I—project established by the Finnish Centre for Nanocellulosic Technologies.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10570-017-1494-4.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Karppinen, A., Vesterinen, AH., Saarinen, T. et al. Effect of cationic polymethacrylates on the rheology and flocculation of microfibrillated cellulose. Cellulose 18, 1381–1390 (2011). https://doi.org/10.1007/s10570-011-9597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-011-9597-9