Abstract
In the present work, amino functionalized nanofibrillated cellulose (NFC) was prepared using click-chemistry in aqueous reaction conditions. First, reactive azide groups were introduced on the surface of NFC by the etherification of 1-azido-2,3-epoxypropane in alkaline water/isopropanol-mixture at ambient temperature. Then the azide groups were reacted with propargyl amine utilizing copper catalyzed azide-alkyne cycloaddition (CuAAC), leading to pH-responsive 1,2,3-triazole-4-methanamine decorated NFC. The reaction products were characterized using Fourier transform infrared spectroscopy, elemental analysis and X-ray photoelectron spectroscopy. The presence of the attached azide groups was also confirmed by reacting them with 5-(dimethylamino)-N-(2-propyl)-1-naphthalenesulfonamide by CuAAC, yielding highly fluorescent NFC. In addition, atom force microscopy and rheology studies confirmed that the original NFC nanostructure was maintained during the synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polysaccharides are very abundant biological raw materials. Nature has developed them to have sophisticated features playing a special role in living organisms. For instance, wood consists of very strong nanostructures that have a large surface area (Nishino et al. 1995). However, the utilization of these properties for different applications faces some challenges, such as the tendency of polysaccharides to absorb water. This becomes a problem when these natural polymers have to be chemically tailored, for example by attaching molecules to their backbone, in order to add specific functionalities to them. Water restricts the chemical reactions available for these modifications, since many of them require dry reaction conditions. Drying of the polysaccharides or using problematic solvents to do the chemical alteration is neither economical nor environmentally benign.
The concept of “Click”-chemistry implies using robust reactions that have high tolerance towards oxygen and water and also work at ambient reaction temperatures, avoiding multiple reaction and purification steps (Kolb et al. 2001). The attraction of these principles has led to the utilization of “click”-reactions in different fields from drug discovery to materials sciences. The copper-catalyzed azide-alkyne cycloaddition (CuAAC) is one of the most used “click”-reaction employed in polymer synthesis, offering extensive possibilities to tailor polymer properties (Binder and Sachsenhofer 2007, 2008; Fournier et al. 2007; Meldal 2008; Rostovtsev et al. 2002; Tornoe et al. 2002). Click-chemistry has also gained attention in the modification of polysaccharides and several articles on the topic can be found (Bernard et al. 2008; De Geest et al. 2008a, b; Hafrén et al. 2006; Hasegawa et al. 2006; Koschella et al. 2010; Krouit et al. 2008; Liebert et al. 2006; Schatz et al. 2009; Tankam et al. 2007; Zhang et al. 2009; Zhao et al. 2010).
Several studies have been made of the chemical modification of nano- and microfibrillated cellulose (Stenstad et al. 2008; Lu et al. 2008) as well as cellulose micro- and nanocrystals and whiskers (Araki et al. 2001; Eyholzer et al. 2010; Dong and Roman 2007; Goussé et al. 2002; Kloser and Gray 2010; Siqueira et al. 2010). However, only one paper exists on the application of click-chemistry on the modification of cellulose nanostructures (Filpponen and Argyropoulos 2010).
In our previous publication, we described a method for introducing azide-groups on the backbone of dextran using aqueous reaction media. In this paper, the method is extended to the surface functionalization of nanofibrillated cellulose. The azide functionalities provide a combinatorial approach to discovering new materials, as a wide range of possible modifications via CuAAC become available. Our aim was to provide a practical aqueous-phase route to azide-containing NFC. As an example, the azide groups were used for producing fluorescent labeled and 1,2,3-triazole-4-methanamine decorated NFC via CuAAC. The large surface area and high aspect ratio of NFC together with the 1,2,3-triazole-4-methanamine functionalities, leads to a material that could be interesting in the research of e.g., catalyst carriers or nanocomposites (Bergbreiter et al. 2007; Chan et al. 2004; Mindt et al. 2006; Suijkerbuijk et al. 2007).
Experimental
Materials
HNO3 (65%) and dansyl chloride (99%) were obtained from Fluka Chemicals and used as received. Propargyl amine (98%), l-ascorbic acid (99%), CuSO4·5H2O (99%), epichlorohydrin (99%), ninhydrin (95%), diethylenetriamine (99%), 2-propanol (99.8%) and NaNO2 (97%) were purchased from Sigma–Aldrich and used as received. NaN3 (99%), acetic acid (100%) and NaOH (99%) were purchased from Merck and used as received.
Nanofibrillated cellulose was obtained from The Finnish Centre for Nanocellulosic Technologies as a dilute hydrogel (solid content 1.66%, with a xylan content of 25%). The sample was prepared by mechanical disintegration of bleached birch pulp by ten passes through a M7115 Fluidizer (Microfluidics Corp. Newton, MA, USA).
Preparation of 1-azido-2,3-epoxypropane
The synthesis of 1-azido-2,3-epoxypropane was done starting from epichlorohydrin. The ring-opening reaction of the epoxide with azide-ion was done according to a slightly modified method (Fringuelli et al. 1999; Pahimanolis et al. 2010). Isopropanol (109.0 mL) and acetic acid (7.2 mL, 125.8 mmol) were mixed with a solution of NaN3 (8.177 g, 125.8 mmol) in 74.0 mL of water. Epichlorohydrin (6.6 mL, 84.2 mmol) was then added under stirring and the reaction was continued at 30 °C for 21 h, until 1H- and 13C-NMR analysis showed complete consumption of the epoxide. A water solution of NaNO2 (11.5 mL, 41.6 mmol) was then added, followed by the dropwise addition of HNO3 (5.76 mL, 83.8 mmol) to eliminate any excess azide-ions. The stirring was continued until the formation of nitrous oxides ceased. The obtained solution of 1-azido-3-chloropropanol (yield 100% by 1H- and 13C-NMR analysis) was stored in dark at room temperature and used without further purification.
Warning! Low molecular weight organic azides are known to be potentially explosive. For this reason, handling concentrated solutions of these materials should be avoided.
The conversion of 1-azido-3-chloropropanol to 1-azido-2,3-epoxypropane was done just prior to use, by adding 26.3 mL of 5 M NaOH to the prepared 1-azido-3-chloropropanol-solution and stirring the mixture for 10 min. The obtained epoxide-solution (yield 100% by 1H- and 13C-NMR analysis) was immediately used for the azide-functionalization of NFC.
Introducing azide groups to the surface of NFC
The azide functionalization of NFC was done following a slightly modified method (Pahimanolis et al. 2010) (Table 1, entry D): To a water suspension of never dried NFC (1,000 g, 16.6 g of solid content) 11.0 mL of 5 M NaOH solution was added and the mixture was stirred for 60 min at 30 °C. The freshly prepared solution of 1-azido-2,3-epoxypropane (197 mL, 84.2 mmol) was then added and the reaction was continued for 24 h at 30 °C, Ph = 12, until 1H-NMR analysis showed complete consumption of the epoxide. The suspension was then purified with deionized water by several centrifugation (20,000×g for 20 min) and redispersion steps until the pH of the suspension became neutral. The obtained azide functionalized NFC was stored in dark at room temperature for further use.
Introducing primary amino groups to NFC using CuAAC
The introduction of 1,2,3-triazole-4-methanamine groups was done as follows (Table 3 entry D-Amine): Propargyl amine (0.300 g, 5.4 mmol) was added to the suspension of azido-NFC (Table 1, entry D, 446 g, 1.03% solids content). A freshly prepared solution of CuSO4·5H2O (0.129 g, 0.52 mmol) and ascorbic acid (AAc) (0.181 g, 1.03 mmol) in 2 mL of water was then added, yielding an immediate bright yellow color. The reaction was carried out for 15 min at 30 °C, after which diethylenetriamine (0.223 g, 2.16 mmol) was added to the reaction mixture in order to complexate the copper ions. The suspension was stirred for another 30 min and by this time the color of the suspension turned from yellow to light blue. The suspension was then purified with deionized water by several centrifugation (20,000×g for 20 min) and redispersion steps, until the supernatant became neutral and no amines were detected with the ninhydrin test.
Labeling of azide functionalized NFC with 5-(dimethylamino)-N-(2-propyl)-1-naphthalenesulfonamide using CuAAC
5-(dimethylamino)-N-(2-propyl)-1-naphthalenesulfonamide was prepared following a reported procedure (Bolletta et al. 1996): Dansyl chloride (0.864 g, 3.2 mmol) was dissolved in 10 mL of anhydrous dichloromethane, followed by the addition of triethylamine (0.55 mL, 4.0 mmol) and propargylamine (0.275 mL, 4.0 mmol). The reaction mixture was stirred at 22 °C for 4 h under argon atmosphere, followed by quenching with 3 × 50 mL of deionized water. The organic phase was dried with MgSO4, filtered and evaporated in vacuum, yielding 5-(dimethylamino)-N-(2-propyl)-1-naphthalenesulfonamide as a yellow syrup (0.720 g, 78%). 1H and 13C-NMR analysis are in accordance with published data.
The fluorescence labeling of azido-NFC was done as follows: 5-(dimethylamino)-N-(2-propyl)-1-naphthalenesulfonamide (0.022 g, 0.076 mmol) was dissolved in 5.0 mL of acetone. A freshly prepared solution of CuSO4·5H2O (0.017 g, 0.068 mmol) and ascorbic acid (AAc) (0.030 g, 0.17 mmol) in 1.0 mL of water was added, resulting in immediate yellow turbid mixture. A sample of a dried azido-NFC sheet (Table 1, entry D) was then immersed in the reaction mixture for 5 min, after which the sample was removed, rinsed with acetone and water, and dried at room temperature. This procedure was also applied to azido-NFC-samples without the addition of the copper catalyst and to unmodified NFC-samples with and without the copper catalyst.
Characterization
1H- and 13C-NMR spectra were recorded on a Varian Gemini 2000 300 MHz spectrometer in deuterium oxide (D2O) or deuterated chloroform (CDCl3). A pulse width of 13.1 μs (90°), relaxation delay of 10 s and acquisition time of 3 s were used for 1H-NMR. For quantitative 13C-NMR, a 90° pulse width of 18.0 μs was used, the relaxation delay and acquisition time being 6 and 1.8 s. Five hundred scans were accumulated for each sample, and the decoupler was gated on only during acquisition, in order to suppress the nuclear Overhauser effect.
Elemental analysis was performed using a Perkin Elmer 2400 Series II CHNS equipment.
The infrared-spectra were obtained with Nicolet Magna IR750 from dried NFC-sheets.
X-ray photoelectron spectroscopy (XPS) analysis was performed using a Kratos AXIS 165 electron spectrometer with monochromatic Al Kα radiation at 100 W and high resolution measurements in carbon C1s and oxygen O1s regions were used. Nitrogen data was collected by trace analysis in the N1s spectra with prolonged acquisition times to enhance the detection limit. Each specimen was measured at three locations.
Acid–base titrations were done using a Philips PW9420 pH-meter equipped with a Hamilton electrochemical sensor (P/N 238000 Hamilton Bonaduz AG, Switzerland). NFC samples were dispersed in deionized water to a solids content of 0.66% (total 30.0 g). One mL of 0.1 M HCl was added and the suspension was stirred for 10 min. The titrations were done at 22 °C with 0.01 M NaOH solution and well reproducible results were obtained.
Atom force microscopy (AFM) images were scanned in tapping mode using a Veeco Dimension 5000 scanning probe microscope with NanoScope V controller and silicon cantilevers. The samples were prepared by placing a drop of dilute NFC suspension (0.005 wt%) on a silica wafer and dried at room temperature overnight.
Rheological measurements were performed using a TA Instruments AR-G2 rheometer equipped with a vane-cup geometry operating at 25 °C (vane diameter 28 mm, vane length 42 mm, cup diameter 30 mm, gap 1 mm). All dynamic viscoelastic measurements were performed at the linear viscoelastic region. This was determined by strain sweep from 0.02 to 10,000% at 1 Hz, and a strain amplitude of 0.5% was chosen. All NFC samples were diluted to a solid content of 1.00 wt% and agitated at 1,400 rpm for 10 min with a propeller mixer. In order to introduce equal shear history, a peak hold step at shear rate of 500 1/s for 30 min was done followed by a time sweep with 1 Hz with 0.5% strain for 2 h in order to recover the structure. The frequency sweeps were performed at 0.02–100 rad/s. Shear rates of 0.01–1,000 1/s were used for shear viscosity studies. The samples were allowed to rest for 10 min between measurements.
Results and discussion
Introduction of azide functionalities to the surface of NFC
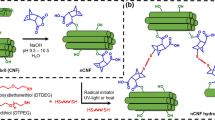
The introduction of azide groups to the surface of NFC was done following a method described for the functionalization of dextran (Pahimanolis et al. 2010). The 1-azido-2,3-epoxypropane was prepared in an one-pot synthesis procedure starting with the ring-opening of epichlorohydrin with azide-ion in the presence of acetic acid. The 1-azido-3-chloropropanol gained was in turn converted to the epoxide-form with alkaline treatment in high yield (Fringuelli et al. 1999). Further, the etherification reaction of 1-azido-2,3-epoxypropane with the surface hydroxyl groups of NFC was carried out under alkaline conditions (Fig. 1). A similar method has recently been reported for the cationization of nanocrystalline cellulose with hydroxypropyltrimethylammonium chloride (Hasani et al. 2008) and it is also commonly employed for the hydroxypropylation of polysaccharides e.g., starch, using epoxypropane (Tomasik and Schilling 2004; Tuschhoff 1986). In this way, azide-groups necessary for the subsequent CuAAC-reaction were introduced in a simple one step reaction, without solvent-exchange or drying steps involved in the synthesis. Because low-molecular weight organic azides are potentially explosive substances, the obtained 1-azido-2,3-epoxypropane solution was used for the etherification reaction without any purification or concentration.
The effect of the amount of added NaOH to the obtained degree of substitution (DS) for the azide groups in NFC is shown in Table 1. The etherification reaction does not occur with low sodium hydroxide to anhydroglucose unit ratio (NaOH/AGU), which may be attributed to insufficient activation of hydroxyl groups on the surface of NFC and therefore low reactivity towards the epoxide (Hasani et al. 2008; Pahimanolis et al. 2010). Using higher NaOH/AGU ratios yields slightly higher azide functionalization. The addition of higher amounts of epoxide also increases the obtained DS value, however, it becomes more difficult to obtain DS values above 0.01, since a large excess of the epoxide has to be used. It should be noted though, that the epoxide solution contains isopropanol, sodium acetate, NaCl and NaNO3 from the preparation of 1-azido-2,3-epoxypropane, which may have some effect on the reaction outcome.
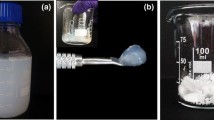
The possibility for some fraction of highly substituted fibrils or hemicellulose to have become solubilized or peeled from the surface during the reaction (Eyholzer et al. 2010; Goussé et al. 2002, 2004) can not be excluded. However, NMR-analysis of samples taken from the purification water did not reveal any peaks characteristic to polysaccharides, indicating that the peeling effect might not be significant, at least in these conditions. Also, AFM images do not reveal notable changes in the morphology of NFC samples (Fig. 2b, c).
The atomic composition of the surfaces of the NFC samples was investigated using XPS while the overall atomic composition was determined with elemental analysis. The data is given in Table 2.
According to the XPS data, the surface nitrogen contents of the azide functionalized samples were much lower than expected, and also clearly lower than the overall nitrogen content determined from the elemental analysis. One explanation for this could be an uneven distribution of functionalities on the surface. Nitrogen was detectable in XPS only with the help of regional trace measurements with much extended acquisition times. However, the surface atomic concentration of N1s was correlating with the azide treatment, rising from the 0.15 at% in the reference to 0.39 at% in the D-amine sample. This increase in nitrogen due to the treatment was much larger in the elemental analysis of overall nitrogen content. Furthermore, the amount of nitrogen found in the reference sample was much lower in the elemental analysis than in XPS.
Low but detectable amounts of surface nitrogen have been observed several times on pure cellulosic specimens when trace measurements have been performed. Together with the elemental analysis this suggests that in the case of the reference sample, the nitrogen is enriched on the topmost surface region only. Apart from the cellulosic components, the nanofibrillated reference sample was found to have elevated aliphatic C–C components in the high resolution XPS measurements. This has actually been the case for all the published XPS nanocellulose studies (Andresen et al. 2006, 2007; Uschanov et al. 2011; Littunen et al. 2011). This phenomenon seems to be connected with the route used in drying nanofibrillar cellulose materials. In the present study, the surface modification was done prior to the drying of the material, meaning that drying would not interfere with the reaction. However, if non-cellulosic carbon species would be accumulating onto the surface of the functionalized cellulose as it does in the case of unmodified cellulose, it would also cover the nitrogen species. This could explain the low surface nitrogen contents observed.
The progress of the etherification reaction was studied by FT-IR, and spectra of samples taken from the reaction mixture at different time points (Table 1, entry D) are shown in Fig. 3a, b and c. A growing peak at 2,110 cm−1 belonging to the azide group indicates that the etherification reaction takes place. The presence of azide groups on NFC was also visualized by reacting them with an alkyne-functionalized fluorescent probe, 5-(dimethylamino)-N-(2-propyl)-1-naphthalenesulfonamide, using the CuAAC-reaction (Fig. 4b). Immersing a dried azide functionalized NFC sheet in a solution containing both the probe and the copper catalyst yielded green–yellow fluorescent NFC with excitation and emission maxima at around 400 and 460 nm, respectively (Fig. 2a). No fluorescent NFC could be obtained without the copper catalyst or when unmodified NFC sheets where used, indicating that the CuAAC-reaction is responsible for the fluorescence labeling.
Introducing primary amino groups to the surface of NFC using CuAAC
The azide functionalized NFC (Table 1, entries D and E) were used to introduce amine groups using the CuAAC-reaction (Fig. 4a). The reaction conditions are given in Table 3. The reaction appeared to be fully quantitative at ambient reaction conditions, since FT-IR analysis showed complete disappearance of the azide peak at 2,110 cm−1 (Fig. 3 spectrum D). Moreover, elemental analysis of the products (Table 2, entries D-Amine and E-Amine) showed an approximately 30% increase in the amount of nitrogen atoms as would be expected from the complete reaction of the azide with propargyl amine. Again, the peeling effect of polysaccharide fragments can not be excluded, however, the purification water of the modified NFC after the centrifugation steps was analyzed with 1H- and 13C-NMR and no polysaccharide fragments were observed. The relatively short reaction time may also have diminished the possible degradation of celluloses by the copper catalyst (Lallana et al. 2009).
The presence of amine groups was confirmed by the titration curve of the suspension (Fig. 5), which shows a buffering effect at the basic region, due to the deprotonation of the ammonium groups. The overall degree of substitution (DS) was determined by the consumption of NaOH solution necessary to neutralize HCl and deprotonate the amines. The amount of amines was found to be approximately 0.15 mmol/g (cellulose) corresponding to DS = 0.02. This value suggests that the nitrogen content found with XPS and elemental analysis are both too low (Table 2, entry D-amine), possibly due to uneven distribution of functionalities or the accumulation of non-cellulosic carbon species onto the surface of NFC upon drying as already mentioned.
Titration curves of 1,2,3-triazole-4-methanamine functionalized NFC (Table 3, entry D-Amine) and unmodified NFC
Interestingly the transformation from azide to amine appears to have an effect on the rheological behavior of the NFC suspension (Fig. 6). All suspensions have a typical decreasing viscosity with increasing shear rate and, at rest, a gel-like behavior with storage modulus G′ being higher than loss modulus G′′ (Agoda-Tandjawa et al. 2010; Pääkkö et al. 2007). The azide functionalized NFC has the lowest storage and loss moduli together with the lowest shear viscosity. We found that subjecting the NFC suspension to similar reaction conditions (as in Table 1, entry D) without the epoxide induced also reduced viscosity and lower moduli. Apparently, the presence of NaOH, sodium acetate, NaCl and NaNO3 and even the centrifugation steps alone may cause aggregation of nanofibrils. It is also possible, that a small fraction of nanosized fibrils might have been lost with the supernatant, which is observed as weaker gel properties. However, when amine-groups are introduced to the surface of NFC via the CuAAc reaction, the moduli and viscosity rise again, close to those of untreated NFC, possibly indicating some recovery of the original structure. The moduli did not increase when the salt treated NFC suspension without azide functionalities was further subjected to the CuAAc reaction conditions (as in Table 3, entry D-Amine).
Viscosity as a function of shear rate (left) and storage modulus (G′, hollow symbols) and loss modulus (G″, filled symbols) as a function of angular frequency (right) for 1.00 wt% suspensions. (open circle, filled circle) Unmodified NFC, (open diamond, filled diamond) unmodified NFC after centrifugation treatment, (open square, filled square) azide functionalized NFC (Table 1, entry D) and (open inverted triangle, filled inverted triangle) 1,2,3-triazole-4-methanamine functionalized NFC (Table 3, entry D-amine)
The effects on the rheological properties by the addition of small amounts of acetic acid or NaOH to the suspensions of unmodified and modified NFC are shown in Fig. 7. In the case of unmodified and azide functionalized NFC, the addition of acid slightly increases the viscosity and moduli of the suspensions. A small increase in ionic strength by the dissociation of the weak acid leads to a moderate screening of the electrostatic repulsions between fibrils (Agoda-Tandjawa et al. 2010, Ono et al. 2004), which would allow for increased interfibrillar friction and thus an increase in the stiffness of the system. On the other hand, the addition of acetic acid to the suspension of 1,2,3-triazole-4-methanamine functionalized NFC, results in lower viscosity and a dramatic drop in moduli. It can be speculated, that the acetic acid builds up near the basic surface of amino functional NFC, affecting the interactions between fibrils, causing the collapse of the fibril network. For comparison, the addition of acetic acid to the reference NFC suspension treated in the CuAAc reaction conditions (as in Table 3, entry D-Amine), results in a small increase in viscosity and moduli. Adding NaOH to the suspension of unmodified or amino functional NFC results in a small drop in moduli indicating a contraction of fibril network. However, the addition of base to azide-functional NFC slightly increases the moduli.
The effect of added AcOH or NaOH on the rheological properties of NFC. At left, viscosity as a function of shear rate, at right storage modulus (G′, hollow symbols) and loss modulus (G″, filled symbols) as a function of angular frequency for 1.00 wt% suspensions. (Open circle, filled circle) no acid or base, (open square, filled square) AcOH concentration 1.8 mmol/L, pH = 5 (open inverted triangle, filled inverted triangle) NaOH concentration 1.8 mmol/L, pH = 11
Conclusions
A simple, aqueous phase one-step synthesis route to prepare azide decorated nanofibrillated cellulose (NFC) is presented. The azide functionalized NFC is a valuable intermediate for broad modification possibilities via the copper catalyzed azide-alkyne cycloaddition (CuAAc), often referred as “click” reaction. The azide groups were further reacted with propargyl amine by CuAAc, yielding 1,2,3-triazole-4-methanamine functionalized, pH responsive NFC.
FT-IR, elemental analysis and XPS analysis as well as acid–base titration proved the functionalization to be successful. The presence of azide groups could also be visualized by reacting them with 5-(dimethylamino)-N-(2-propyl)-1-naphthalenesulfonamide by CuAAc yielding highly fluorescent NFC. According to rheology studies, the reaction conditions influence the rheological behavior of NFC.
References
Agoda-Tandjawa G, Durand S, Berot S, Blassel C, Gaillard C, Garnier C, Doublier J-L (2010) Rheological characterization of microfibrillated cellulose suspensions after freezing. Carbohydr Polym 80:677–686
Andresen M, Johansson L-S, Tanem B, Stenius P (2006) Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 13:665–677
Andresen M, Stenstad P, Møretrø T, Langsrud S, Syverud K, Johansson L-S, Stenius P (2007) Nonleaching antimicrobial films prepared from surface modified microfibrillated cellulose. Biomacromolecules 8:2149–2155
Araki J, Wada M, Kuga S (2001) Steric stabilization of cellulose microcrystal suspension by poly (ethylene glycol) grafting. Langmuir 17:21–27
Bergbreiter D, Hamilton P, Koshti N (2007) Self-separating homogeneous copper(I) catalysts. J Am Chem Soc 129:10666–11667
Bernard J, Save M, Arathoon B, Charleux B (2008) Preparation of a xanthate-terminated dextran by click-chemistry: application to the synthesis of polysaccharide-coated nanoparticles via surfactant-free ab initio emulsion polymerization of vinyl acetate. J Polym Sci Part A: Polym Chem 46:2845–2857
Binder W, Sachsenhofer R (2007) ‘Click’ chemistry in polymer and materials science. Macromol Rapid Commun 28:15–54
Binder W, Sachsenhofer R (2008) ‘Click’ chemistry in polymer and materials science: an update. Macromol Rapid Commun 29:952–981
Bolletta F, Fabbri D, Lombardo M, Prodi L, Trombini C, Zaccheroni N (1996) Synthesis and photophysical properties of fluorescent derivatives of methylmercury. Organometallics 15:2415–2417
Chan T, Hilgraf R, Sharpless K, Fokin V (2004) Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org Lett 6:2853–2855
De Geest B, Van Camp W, Du Prez F, De Smedt S, Demeester J, Hennink W (2008a) Biodegradable microcapsules designed via ‘click’ chemistry. Chem Commun (2):190–192
De Geest B, Van Camp W, Du Prez F, De Smedt S, Demeester J, Hennink W (2008b) Degradable multilayer films and hollow capsules via a ‘click’ strategy. Macromol Rapid Commun 29:1111–1118
Dong S, Roman M (2007) Fluorescently labelled cellulose nanocrystals for bioimaging applications. J Am Chem Soc 129:13810–13811
Eyholzer CH, Bordeanu N, Lopez-Suevos F, Rentsch D, Zimmermann T, Oksman K (2010) Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 17:19–30
Filpponen I, Argyropoulos DS (2010) Regular linking of cellulose nanocrystals via click chemistry: synthesis and formation of cellulose nanoplatelet gels. Biomacromolecules 11:1060–1066
Fournier D, Hoogenboom R, Schubert U (2007) Clicking polymers: a straightforward approach to novel macromolecular architectures. Chem Soc Rev 36:1369–1380
Fringuelli F, Piermatti O, Pizzo F, Vaccaro L (1999) Ring opening of epoxides with sodium azide in water. A regioselective pH-controlled reaction. J Org Chem 64:6094–6096
Goussé C, Chanzy H, Excoffier G, Soubeyrand L, Fleury E (2002) Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvents. Polymer 43:2645–2651
Goussé C, Chanzy H, Cerrada ML, Fleury E (2004) Surface silylation of cellulose microfibrils: preparation and rheological properties. Polymer 45:1569–1575
Hafrén J, Zou W, Córdova A (2006) Heterogeneous ‘organoclick’ derivatization of polysaccharides. Macromol Rapid Commun 27:1362–1366
Hasani M, Cranston E, Westman G, Gray D (2008) Cationic surface functionalization of cellulose nanocrystals. Soft Matter 4:2238–2244
Hasegawa T, Umeda M, Numata M, Li C, Bae A-H, Fujisawa T, Haraguchi S, Sakurai K, Shinkai S (2006) ‘Click chemistry’ on polysaccharides: a convenient, general, and monitorable approach to develop (1 --> 3)-beta-d-glucans with various functional appendages. Carbohydr Res 341:35–40
Kloser E, Gray DG (2010) Surface grafting of cellulose nanocrystals with poly(ethylene oxide) in aqueous media. Langmuir 26:13450–13456
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004–2021
Koschella A, Richter M, Heinze T (2010) Novel cellulose-based polyelectrolytes synthesized via the click reaction. Carbohydr Res 345:1028–1033
Krouit M, Bras J, Belgacem N (2008) Cellulose surface grafting with polycaprolactone by heterogeneous click-chemistry. Eur Polym J 44:4074–4081
Lallana E, Fernandez-Megia E, Riguera R (2009) Surpassing the use of copper in the click functionalization of polymeric nanostructures: a strain promoted approach. J Am Chem Soc 131:5748–5750
Liebert T, Hänsch C, Heinze T (2006) Click chemistry with polysaccharides. Macromol Rapid Commun 27:208–213
Littunen K, Hippi U, Johansson L-S, Österberg M, Tammelin T, Laine J, Seppälä J (2011) Free radical graft copolymerization of nanofibrillated cellulose with acrylic monomers. Carbohydr Polym 84:1039–1047
Lu J, Askeland P, Drzal LT (2008) Surface modification of microfibrillated cellulose for epoxy composite applications. Polymer 49:1285–1296
Meldal M (2008) Polymer “clicking” by CuAAC reactions. Macromol Rapid Commun 29:1016–1051
Mindt T, Struthers H, Brans L, Anguelov T, Schweinsberg C, Maes V, Tourwé D, Schibli R (2006) “Click to chelate”: synthesis and installation of metal chelate into biomolecules in a single step. J Am Chem Soc 128:15096–15097
Nishino T, Takano K, Nakamae K (1995) Elastic modulus of the crystalline regions of cellulose polymorphs. J Polym Sci, Part B: Polym Phy 33:1647–1651
Ono H, Shimaya Y, Sato K, Hongo T (2004)1H spin–spin relaxation time of water and rheological properties of cellulose nanofiber dispersion, transparent cellulose hydrogel (TCG). Polym J 36(9):684–694
Pääkkö M, Ankerfors M, Kosonen H, Nykanen A, Ahola S, Österberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindström T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941
Pahimanolis N, Vesterinen A-H, Rich J, Seppala J (2010) Modification of dextran using click-chemistry approach in aqueous media. Carbohydr Polym 82:78–82
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed 41:2596–2599
Schatz C, Louguet S, Le Meins J-F, Lecommandoux S (2009) Polysaccharide-block-polypeptide copolymer vesicles: towards synthetic viral capsids. Angew Chem Int Ed 48:2572–2575
Siqueira G, Bras J, Dufresne A (2010) New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 26:402–411
Stenstad P, Andresen M, Tanem BS, Stenius P (2008) Chemical surface modifications of microfibrillated cellulose. Cellulose 15:35–45
Suijkerbuijk M, Aerts B, Dijkstra H, Lutz M, Spek A, Koten G, Gebbink R (2007) “Click” 1,2,3-triazoles as tunable ligands for late transition metal complexes. Dalton Trans (13):1273–1276
Tankam P, Müller R, Mischnick P, Hopf H (2007) Alkynyl polysaccharides: synthesis of propargyl potato starch followed by subsequent derivatizations. Carbohydr Res 342:2049–2060
Tomasik P, Schilling C (2004) Chemical modification of starch. Adv Carbohydr Chem Biochem 59:175–403
Tornoe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes–azides. J Org Chem 67:3057–3064
Tuschhoff JV (1986) Hydroxypropylated starches. In: Wurzburg OB (ed) Modified starches: properties and uses. CRC Press, Boca Raton, pp 89–96
Uschanov P, Johansson L-S, Maunu SL, Laine J (2011) Heterogeneous modification of various celluloses with fatty acids. Cellulose 18(2):393–404
Zhang J, Xu X-D, Wu D-Q, Zhang X-Z, Zhuo R-X (2009) Synthesis of thermosensitive P (NIPAAm-co-HEMA)/cellulose hydrogels via “click” chemistry. Carbohydr Polym 77:583–589
Zhao G-L, Hafrén J, Deiana L, Górdova A (2010) Heterogeneous “organoclick” derivatization of polysaccharides: photochemical thiol-ene click modification of solid cellulose. Macromol Rapid Commun 31:740–744
Acknowledgments
This work has been funded by the Graduate School for Biomass Refining (Academy of Finland) and the Finnish Funding Agency for Technology and Innovation (project “Tailoring of nanocellulose structures for industrial applications” NASEVA). We gratefully acknowledge Dr. Joseph M. Campbell for his contribution to the XPS measurements and Ms. Arja-Helena Vesterinen, Mr. Matti Juhani Pusa and Dr. Antti Laukkanen for comments and scientific discussions. Ms. Tiia Juhala is acknowledged for the elemental analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found online at http://dx.doi.org/10.1007/s10570-017-1500-x.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pahimanolis, N., Hippi, U., Johansson, LS. et al. Surface functionalization of nanofibrillated cellulose using click-chemistry approach in aqueous media. Cellulose 18, 1201–1212 (2011). https://doi.org/10.1007/s10570-011-9573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-011-9573-4