Abstract
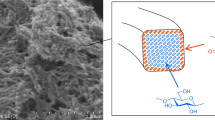
In this study, we developed a surface-activation technique for cellulose nanofibrils (CNFs) using mild-alkali and aqueous conditions. CNFs were initially processed using the aqueous counter collision (ACC) method to produce Janus-type amphiphilic CNFs with both hydrophilic and hydrophobic faces on the surface of a single nanofibril (ACC-CNF). Selective functionalization of the hydroxy groups on the hydrophilic faces creates an opportunity to develop novel nano-building blocks that introduce heterogeneous and tailored surface characteristics into the design of nanomaterials. In this study, alkaline conditions were used to activate the hydroxy groups on the surface of ACC-CNFs as a pre-treatment for the partial crystalline transformation from cellulose I to cellulose II. We found that alkali treatment with sodium hydroxide (NaOH) solutions (concentration range 1–7 wt%) did not fully transform the structure of ACC-CNFs into cellulose II, nor change the morphology of nanofibrils, as seen from their wide-angle X-ray diffraction patterns and atomic force microscopy images. We also found that the hydroxy groups at the surface region of the ACC-CNFs were sufficiently reactive under the moderate alkali and aqueous conditions to undergo subsequent carboxymethylation. Therefore, alkali treatment of ACC-CNFs with a 1–7 wt% NaOH solution rendered the surface of the ACC-CNFs as sufficiently reactive for chemical modification without morphological changes. This simple method for surface activation of CNFs can be useful in the development of future sustainable and novel materials for a variety of applications.
Similar content being viewed by others
Introduction
In nature, the hierarchical self-organization of molecular or nano-scale building blocks can result in the formation of three-dimensional structures with unique properties and functions [1, 2]. Synthetic amphiphilic substances, including low molecular weight surfactants [3], polymers [4] and nano-objects [5] can also be fabricated into functional materials with higher ordered structures. The interfacial interaction between the building blocks is an important factor in the bottom-up synthesis of these higher ordered structures. In particular, the surface properties of the building blocks should be closely related to those of the target hierarchical structure to successfully fabricate.
Cellulose microfibrils are important building blocks in nature and are integral in the formation of plant cell walls. Nowadays, cellulose microfibrils and their bundles are capable to be extracted as cellulose nanofibrils (CNFs) by methodological developments of nano-refining of pulp fibers [6]. The unique properties of CNFs, with their large specific surface areas [6], mechanical properties [7] and thermal stabilities [8], and their natural role as a bio-based nano-building block, make them suitable materials in the fabrication of functional higher order structures.
Kondo et al. developed the aqueous counter collision (ACC) method as a nano-pulverization process [9, 10] that extracts nano-objects from a variety of natural resources. When raw cellulosic material in an aqueous suspension is subjected to the ACC method, specific intermolecular interactions are cleaved by the water collision energy propagated throughout the sample, generating single CNFs (ACC-CNFs) dispersed in water [10, 11]. The size and morphology of the resulting ACC-CNFs is dependent on the starting raw material [10,11,12]. Using the ACC method ensures that both hydrophilic and hydrophobic planes, which are contained in natural cellulose crystals, are exposed on the surface of single ACC-CNFs [13]. Therefore, ACC-CNFs exhibit unique “Janus-type” amphiphilic surface properties [13], including switchable surface wettability [14], functioning that facilitates the formation of Pickering emulsions [12, 15] and adsorption onto hydrophobic polymer particles [16]. We recently reported that the surface properties of ACC-CNFs can be altered via a simple process using oil droplets in a Pickering emulsion as a reaction platform [17, 18]. In this way, the surface amphiphilicity of the initial ACC-CNFs can be controlled to fabricate higher order architectures with novel functionalities.
Generally, the surfaces of crystalline cellulose are chemically resistant [19] and therefore, surface activation of the CNFs is required to add further functionality. Ideally, surface activation should be achieved using an environmentally friendly process with low energy requirements and non-toxic reagents. Ball milling is a commonly used approach for activating and chemically modifying the surfaces of cellulose fibers in a solvent-free system [20, 21]. For aqueous dispersions, alkali treatments can enhance the chemical reactivity of cellulosic materials by removing hemicellulose and lignin, as well as by altering the crystallinity and the sporadic breaking of hydrogen bonds [20, 22, 23].
We have focused on the prospect of ACC-CNFs having unique surface properties as a nano-building block. In the initial step for facile modification of the surface, this study attempts to activate the hydroxy group on the surface of ACC-CNFs while maintaining their crystallinity of the fiber shape under moderate alkaline conditions using low concentrations of aqueous sodium hydroxide (NaOH). Then, carboxymethylation, a method for ether derivatization, was conducted as an example of post-chemical modification of ACC-CNFs.
Experimental
Materials
The starting material for preparing the ACC-CNFs was microcrystalline cellulose (MCC, Funacel II®, Funakoshi Co., Ltd., Tokyo, Japan). MCC powder was rinsed with excess water and ethanol prior to ACC treatment, as implemented in previous studies [15, 24]. NaOH, chloroacetic acid and hydrochloric acid were purchased from FUJIFILM Wako Pure Chemical Corp., Osaka, Japan. Potassium bromide (KBr) was purchased from Sigma-Aldrich Japan LLC., Tokyo, Japan. All chemical reagents were used as received without further purification. Deionized (DI) water was used for all experimental procedures.
Preparation of cellulose nanofibrils (ACC-CNFs)
An aqueous suspension of pre-rinsed MCC (1.0 wt%) was treated using an ACC system (CNNT Co., Ltd., Incheon, South Korea) equipped with nozzles 160 μm in diameter. The ACC treatment was repeated for 60 cycles at an ejecting pressure of 200 MPa. To remove micrometer-scale residues, the concentration of the ACC-CNF dispersion was diluted to 0.4 wt% and then centrifuged (370×g, 10 min, 25 °C [10]).
Alkali treatment of ACC-CNFs
All dispersions and solutions were heated at 60 °C for 30 min prior to mixing. Typically, 10 g of an aqueous ACC-CNF dispersion (0.2 wt%) was mixed with 5 g of an aqueous NaOH solution. The mixtures were then stirred at. As a result, the final concentrations of NaOH in the aqueous mixtures ranged from of 1–20 wt%. The initial MCC powders prior to ACC treatments (0.02 g) were stirred in 1–20 wt% NaOH aqueous solutions at 60 °C for 1 h.
Surface carboxymethylation
The alkali-treated ACC-CNFs were collected as a precipitate by centrifugation (1500×g, 10 min, 25 °C). An aqueous solution of chloroacetic acid was added to the still wet samples. The final concentration of chloroacetic acid in the mixture was adjusted to 20 wt%. The mixture was then stirred at 45 °C for 4 h [25]. The reaction media were displaced with DI water by centrifugation (1500×g, 10 min, 25 °C) to obtain the precipitated product.
Wide-angle X-ray diffraction (WAXD) measurement
As outlined above, after each alkali treatment, the alkaline media were exchanged with DI water by repeated centrifugation and re-dispersion, until reaching neutral pH 7.0. Neutralized samples were freeze-dried ready for wide-angle X-ray diffraction (WAXD) measurements (RINT 2000 V or SmartLab, Rigaku Corp., Tokyo, Japan). The WAXD profiles were acquired using Ni-filtered CuKα radiation (λ = 0.1542 nm) at 40 kV and 20 mA in 5–40°of 2θ. The scan rate and step were 0.5–2° min−1 and 0.05°, respectively. Carboxymethylated ACC-CNFs (CM-CNFs) were also measured via WAXD under the same conditions.
Peak separation of the WAXD profiles [26,27,28] was conducted based on the second derivative of the profile data using the spectral data processing software GRAMS/AI (Thermo Fischer Scientific, Inc. USA), prior to the following calculations. The crystallinity and the extent of transformation into cellulose II (Cellulose II ratio (%)) were calculated from the WAXD profiles (n = 3–5) according to previous references [27, 29,30,31]:
Fourier transform infrared (FT-IR) spectroscopy
To undergo FT-IR measurements, the CM-CNFs were lyophilized and embedded into KBr pellets. Measurements were performed using an FT-IR spectrophotometer equipped with a TGS detector (FT/IR-620, JASCO Corp., Tokyo, Japan). The accumulation number, resolution and wavenumber region of the measurements were 32 scans, 2 cm−1 and 400–4000 cm−1, respectively. All spectra were normalized using the band at 1162 cm−1, attributable to the C–O stretching vibration of the cellulose backbone [32].
Atomic force microscopy (AFM)
At each treatment step, the size and morphology of the ACC-CNFs were observed by AFM (MFP-3D Origin; Oxford Instruments Asylum Research, Inc., Santa Barbara, CA, USA) using a silicon cantilever (OMCL-AC160TS-R3; Olympus Corp., Tokyo, Japan). An aqueous dispersion of CNFs (1.0 × 10−3 wt%) was dropped onto a cleaved natural mica substrate (The Nilaco Corp., Tokyo, Japan), then dried at room temperature. The AFM measurements were operated in AC mode under ambient conditions.
Conductivity titration
The quantity of carboxy groups on the surface of the CM-CNFs was estimated using the conductivity titration method [33]. Water dispersions of CM-CNFs were adjusted to a pH of 3 or less using HCl in the presence of NaCl. The titration was performed with a 0.01 M aqueous NaOH solution.
Sedimentation test
Water dispersions of ACC-CNFs and CM-CNFs were adjusted to pH 4, 7 and 10 prior to shaking for 30 min at room temperature. Centrifugation (500×g, 10 min, 25 °C) was used to compare the dispersion stability of each sample.
Results and discussion
Alkali-induced swelling of cellulose nanofibrils
ACC-CNFs were treated with 1–20 wt% aqueous NaOH solutions, and the extent of alkali-induced swelling of the nanofibrils was indirectly estimated from the transformation of the crystalline polymorphs. Figure 1a shows WAXD profiles of the raw materials of ACC-CNFs, namely MCC powders, in a dried state after rinsed with DI water following alkali treatment. The native cellulose I structure of the wood-based MCC was almost unchanged with up to 10 wt% NaOH. The cellulose II structure became dominant as crystal structure by alkali treatment at 12.5 wt% NaOH, while significant cellulose I structure was not observed over 15 wt% NaOH. Figure 1b shows the WAXD profiles of the alkali-treated ACC-CNFs. The original wood-based ACC-CNFs provided the reference diffraction pattern for a native cellulose I structure similar to MCC. Following alkali treatment with up to 10 wt% NaOH, the WAXD profiles show the appearance of shoulders and diffraction peaks attributable to the cellulose II crystal structure. With alkali treatment over 12.5 wt% NaOH, the intensity of these peaks stabilized, indicating that the ACC-CNF crystal structure had transformed from cellulose I to cellulose II. Thus, the structural transformation of ACC-CNFs was implied to be more sensitive to the alkali concentration than the case of microfibers.
Figure 2 shows the crystallinity and the proportion of cellulose II structure in the crystalline region, as calculated from the WAXD profiles of CNFs treated with less than 10 wt% NaOH solution. When the NaOH concentration exceeded 7 wt%, there was a significant decrease in the crystallinity and increase in the proportion of cellulose II structure present. These results indicate that the cellulose I structure at surface regions of the ACC-CNFs was partially transformed into the cellulose II structure [34]. The results also imply that the alkali-penetrated region of ACC-CNFs is sensitive to the NaOH concentration. According to previous studies [20, 23] regarding alkali treatment of plant fibers at low NaOH concentrations (less than ca. 10 wt%), the hydroxide ions cannot fully penetrate the cellulose crystalline lattice because of size restrictions of the hydrated ions. Therefore, transformation of the crystal structure in entire cellulosic fibers is not likely to occur. The partial crystalline transition of ACC-CNFs at low concentrations of NaOH might be due to the large specific surface area of our material [11, 13]. Namely, treatment with 1–5 wt% of NaOH solution promoted conversion of the hydroxy groups into alkoxide groups only at the surface of the ACC-CNFs. Therefore, it was important to investigate the activation effect of the alkali treatment on surface chemical modification of the crystalline ACC-CNFs, as discussed in the following sections.
Carboxymethylation of activated cellulose nanofibrils
FT-IR spectra for the ACC-CNFs after the carboxymethylation reaction are shown in Fig. 3. The absorption band detected at 1603 cm−1 is attributable to the C=O of the carboxylate [35]. The band intensity increased with increasing concentration of the NaOH used during the pre-treatment stage. The quantity of carboxymethyl (CM) groups introduced on the surface of the ACC-CNFs was estimated by a conductivity titration. When the pre-treatment concentration of NaOH was over 3 wt%, the quantity of CM groups increased (Fig. 4). These results suggest that the initial alkali treatment activated the reactivity of the hydroxy groups on the surface of ACC-CNFs, enhancing the introduction efficiency of the CM groups during subsequent carboxymethylation. However, higher concentrations of NaOH (over 10 wt%) caused a significant transition of the crystal structure from cellulose I to cellulose II (see Figs. 1 and 2). Therefore, 3–7 wt% is a suitable alkaline concentration range for the surface activation of the ACC-CNFs. AFM measurements showed that the alkali pre-treatment and carboxymethylation had a negligible influence on the size and morphology of the ACC-CNFs (Fig. 5 and Table 1).
FT-IR spectra of cellulose nanofibrils derivatized through carboxymethylation after alkali-activation with different NaOH concentrations. NaOH concentrations: a 0.0 wt%, b 1.0 wt%, c 3.0 wt%, d 5.0 wt% and e 7.0 wt%, corresponding to Fig. 1b
Sedimentation tests were performed to examine how dependent the introduction of CM groups was on the surface properties of the ACC-CNFs, as shown in Fig. 6. The aqueous dispersions of the untreated ACC-CNFs and CM-ACC-CNFs were centrifuged at 500×g for 10 min at pH 4, 7 and 10. The untreated ACC-CNFs precipitated regardless of the pH (Fig. 6a). In contrast, the CM-ACC-CNFs appeared dispersed under basic conditions (Fig. 6b). This is presumably because of the increase in electrostatic repulsion between the negatively charged carboxylate groups (pKa 4.4) [36]. The aggregation and precipitation of CM-CNFs under acidic conditions are hypothesized to result from the formation of acidic carboxyl groups. These results indicate that the introduction of CM groups to the surface introduces pH-responsive surface properties.
Conclusions
In this study, we investigated a simple and novel approach for the surface activation of ACC-CNFs under mild aqueous and alkaline conditions. Hydroxy groups were activated at the surface of the wood-derived ACC-CNFs through partial swelling by mild alkali treatment with aqueous NaOH solutions. The extent of the subsequent CM derivatization of the ACC-CNF surface was dependent on the alkali-activation conditions, with 3 to 7 wt% found as the optimum alkaline concentration range for surface activation. The introduction of CM groups to the ACC-CNF surface improved their dispersibility in water and simultaneously introduced pH-responsive behavior. This is an exciting example of introducing a dynamic chemically modified surface onto CNFs. Overall, this facile activation treatment allowed for the subsequent chemical modification of the ACC-CNF surface under mild conditions, thus creating new opportunities for ACC-CNFs to be developed into functional bio-based materials.
Availability of data and materials
Not applicable.
Abbreviations
- ACC:
-
Aqueous counter collision
- AFM:
-
Atomic force microscopy
- CM:
-
Carboxymethyl(ated)
- CNFs:
-
Cellulose nanofibrils
- DI:
-
Deionized
- FT-IR:
-
Fourier transform infrared
- MCC:
-
Microcrystalline cellulose
- WAXD:
-
Wide-angle X-ray diffraction
References
Shimizu T (2002) Bottom-up synthesis and structural properties of self-assembled high-axial-ratio nanostructures. Macromol Rapid Commun 23:311–331. https://doi.org/10.1002/1521-3927(20020401)23:5/6%3c311::aid-marc311%3e3.0.co;2-u
Mendes AC, Baran ET, Reis RL, Azevedo HS (2013) Self-assembly in nature: using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:582–612. https://doi.org/10.1002/wnan.1238
Fan YX, Wang YL (2018) Self-assembly and functions of star-shaped oligomeric surfactants. Langmuir 34:11220–11241. https://doi.org/10.1021/acs.langmuir.8b00290
Palivan CG, Goers R, Najer A, Zhang XY, Car A, Meier W (2016) Bioinspired polymer vesicles and membranes for biological and medical applications. Chem Soc Rev 45:377–411. https://doi.org/10.1039/c5cs00569h
Walther A, Muller AHE (2013) Janus particles: synthesis, self-assembly, physical properties, and applications. Chem Rev 113:5194–5261. https://doi.org/10.1021/cr300089t
Isogai A (2013) Wood nanocelluloses: fundamentals and applications as new bio-based nanomaterials. J Wood Sci 59:449–459. https://doi.org/10.1007/s10086-013-1365-z
Saito T, Kuramae R, Wohlert J, Berglund LA, Isogai A (2013) An ultrastrong nanofibrillar biomaterial: the strength of single cellulose nanofibrils revealed via sonication-induced fragmentation. Biomacromol 14:248–253. https://doi.org/10.1021/bm301674e
Nishino T, Matsuda I, Hirao K (2004) All-cellulose composite. Macromolecules 37:7683–7687. https://doi.org/10.1021/ma049300h
Kondo T, Morita M, Hayakawa K, Onda Y (2008) Polysaccharide wet pulverization method. US Patent 7357339-B2, 2008.
Kondo T, Kose R, Naito H, Kasai W (2014) Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr Polym 112:284–290. https://doi.org/10.1016/j.carbpol.2014.05.064
Kose R, Mitani I, Kasai W, Kondo T (2011) “Nanocellulose” as a single nanofiber prepared from pellicle secreted by Gluconacetobacter xylinus using aqueous counter collision. Biomacromol 12:716–720. https://doi.org/10.1021/bm1013469
Tsuboi K, Yokota S, Kondo T (2014) Difference between bamboo- and wood-derived cellulose nanofibers prepared by the aqueous counter collision method. Nord Pulp Pap Res J 29:69–76. https://doi.org/10.3183/npprj-2014-29-01-p069-076
Tsuji T, Tsuboi K, Yokota S, Tagawa S, Kondo T (2021) Characterization of an amphiphilic janus-type surface in the cellulose nanofibril prepared by aqueous counter collision. Biomacromol 22:620–628. https://doi.org/10.1021/acs.biomac.0c01464
Kose R, Kasai W, Kondo T (2011) Switching surface properties of substrates by coating with a cellulose nanofiber having a high adsorbability. Sen-i Gakkaishi 67:163–168. https://doi.org/10.2115/fiber.67.163
Yokota S, Kamada K, Sugiyama A, Kondo T (2019) Pickering emulsion stabilization by using amphiphilic cellulose nanofibrils prepared by aqueous counter collision. Carbohydr Polym 226:115293. https://doi.org/10.1016/j.carbpol.2019.115293
Ishikawa G, Tsuji T, Tagawa S, Kondo T (2021) Adsorption of Janus-type amphiphilic cellulose nanofibrils onto microspheres of semi-crystalline polymers. Macromolecules 54:9393–9400. https://doi.org/10.1021/acs.macromol.1c01163
Ishida K, Yokota S, Kondo T (2021) Localized surface acetylation of aqueous counter collision cellulose nanofibrils using a Pickering emulsion as an interfacial reaction platform. Carbohydr Polym 261:117845. https://doi.org/10.1016/j.carbpol.2021.117845
Yokota S, Tagawa S, Kondo T (2021) Facile surface modification of amphiphilic cellulose nanofibrils prepared by aqueous counter collision. Carbohydr Polym 255:117342. https://doi.org/10.1016/j.carbpol.2020.117342
Brumer H, Zhou Q, Baumann MJ, Carlsson K, Teeri TT (2004) Activation of crystalline cellulose surfaces through the chemoenzymatic modification of xyloglucan. J Am Chem Sco 126:5715–5721. https://doi.org/10.1021/ja0316770
Gallego R, Piras CC, Rutgeerts LAJ, Fernandez-Prieto S, De Borggraeve WM, Franco JM, Smets J (2020) Green approach for the activation and functionalization of jute fibers through ball milling. Cellulose 27:643–656. https://doi.org/10.1007/s10570-019-02831-0
Huang L, Wu Q, Wang QW, Wolcott M (2019) One-step activation and surface fatty acylation of cellulose fibers in a solvent-free condition. ACS Sustain Chem Eng 7:15920–15927. https://doi.org/10.1021/acssuschemeng.9b01974
Jahn A, Schroder MW, Futing M, Schenzel K, Diepenbrock W (2002) Characterization of alkali treated flax fibres by means of FT Raman spectroscopy and environmental scanning electron microscopy. Spectrochim Acta A Mol Biomol Spectrosc 58:2271–2279. https://doi.org/10.1016/s1386-1425(01)00697-7
Liu YP, Hu H (2008) X-ray diffraction study of bamboo fibers treated with NaOH. Fibers Polym 9:735–739. https://doi.org/10.1007/s12221-008-0115-0
Huan SQ, Yokota S, Bai L, Ago M, Borghei M, Kondo T, Rojas OJ (2017) Formulation and composition effects in phase transitions of emulsions costabilized by cellulose nanofibrils and an ionic surfactant. Biomacromol 18:4393–4404. https://doi.org/10.1021/acs.biomac.7b01452
Heydarzadeh HD, Najafpour GD, Nazari-Moghaddam AA (2009) Catalyst-free conversion of alkali cellulose to fine carboxymethyl cellulose at mild conditions. World Appl Sci J 6:564–569
Wang Y, Zhao YL, Deng YL (2008) Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr Polym 72:178–184. https://doi.org/10.1016/j.carbpol.2007.08.003
Garvey CJ, Parker IH, Simon GP (2005) On the interpretation of X-ray diffraction powder patterns in terms of the nanostructure of cellulose I fibres. Macromol Chem Phys 206:1568–1575. https://doi.org/10.1002/macp.200500008
Cao Y, Tan HM (2005) Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzyme Microb Technol 36:314–317. https://doi.org/10.1016/j.enzmictec.2004.09.002
Hult LE, Iversen T, Sugiyama J (2003) Characterization of the supramolecular structure of cellulose in wood pulp fibres. Cellulose 10:103–110. https://doi.org/10.1023/A:1024080700873
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10. https://doi.org/10.1186/1754-6834-3-10
Mansikkamaki P, Lahtinen M, Rissanen K (2007) The conversion from cellulose I to cellulose II in NaOH mercerization performed in alcohol–water systems: an X-ray powder diffraction study. Carbohydr Polym 68:35–43. https://doi.org/10.1016/j.carbpol.2006.07.010
Liang CY, Marchessault RH (1959) Infrared spectra of crystalline polysaccharides. II. Native celluloses in the region from 640 to 1700 cm-1. J Polym Sci 39:269–278. https://doi.org/10.1002/pol.1959.1203913521
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromol 5:1983–1989. https://doi.org/10.1021/bm0497769
Okano T, Sarko A (1985) Mercerization of cellulose. II. Alkali cellulose intermediates and a possible mercerization mechanism. J Appl Polym Sci 30:325–332. https://doi.org/10.1002/app.1985.070300128
Pushpamalar V, Langford SJ, Ahmad M, Lim YY (2006) Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr Polym 64:312–318. https://doi.org/10.1016/j.carbpol.2005.12.003
Song JL, Birbach NL, Hinestroza JP (2012) Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 19:411–424. https://doi.org/10.1007/s10570-011-9647-3
Acknowledgements
We thank Mr. Koichiro Ishida (Kyushu University) for his support measuring the size and morphology of fibers.
Funding
This work was supported by JSPS KAKENHI Grant Numbers 18K05767 and 25712020.
Author information
Authors and Affiliations
Contributions
SY: research plan throughout the study and preparation of the paper; AN: performing experiments; TK: research plan and preparation of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yokota, S., Nishimoto, A. & Kondo, T. Alkali-activation of cellulose nanofibrils to facilitate surface chemical modification under aqueous conditions. J Wood Sci 68, 14 (2022). https://doi.org/10.1186/s10086-022-02022-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-022-02022-9