Abstract
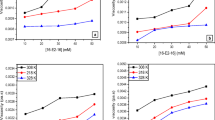
Mixtures of diblock co-oligomers of tri-O-methylated and unmodified cello-oligosaccharides have been found to be amphiphilic, as reported before. In order to clarify their accurate amphiphilic property, diblock co-oligomers of tri-O-methylated and unmodified cello-oligosaccharides with monodispersity, methyl β-d-glucopyranosyl-(1→4)-2,3,6–tri-O-methyl-β-d-glucopyranosyl-(1→4)-2,3,6–tri-O-methyl-β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-methyl-β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-methyl-d-glucopyranoside (1, pentamer), methyl β-d-glucopyranosyl-(1→4)- β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-methyl-β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-methyl-β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-methyl-β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-methyl-d-glucopyranoside (2, hexamer), and methyl β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-methyl-β-d-glucopyranosyl-(1→4)- 2,3,6-tri-O-methyl-d-glucopyranoside (3, trimer) were synthesized independently. These compounds had higher surface activities compared to the mixture of diblock co-oligomers of tri-O-methylated and unmodified cello-oligosaccharides and commercially available methylcellulose (MC) SM-4. This paper describes the methods of synthesis of these compounds, and the influence of amphiphilic character on their surface activity. A new class of carbohydrate-based nonionic surfactant without long alkyl chain was discovered.










Similar content being viewed by others
References
Chang SA, Gray DG (1978) Surface-tension of aqueous hydroxypropyl cellulose solutions. J Colloid Interface Sci 67(2):255–265
Evertsson H, Nilsson S (1998) Microviscosity in dilute aqueous solutions of sds and non-ionic cellulose derivatives of different hydrophobicity: Fluorescence probe investigations. Carbohydr Polym 35(3–4):135–144
Gaonkar AG (1991) Surface and interfacial activities and emulsion characteristics of some food hydrocolloids. Food Hydrocolloids 5(4):329–337
Hato M, Minamikawa H, Tamada K, Baba T, Tanabe Y (1999) Self-assembly of synthetic glycolipid/water systems. Adv Colloid Interface Sci 80(3):233–270
Kamitakahara H, Hori M, Nakatsubo F (1996) Substituent effect on ring-opening polymerization of regioselectively acylated α-d-glucopyranose 1,2,4-orthopivalate derivatives. Macromolecules 29(19):6126–6131
Kamitakahara H, Nakatsubo F, Klemm D (2006) Block co-oligomers of tri-O-methylated and unmodified cello-oligosaccharides as model compounds for methylcellulose and its dissolution/gelation behavior. Cellulose 13(4):375–392
Kamitakahara H, Nakatsubo F, Murakami K (1994) Ring-opening polymerization of 1,4-anhydro-α-d-glucopyranose derivatives having acyl-groups and synthesis of (1→5)-β-d-glucofuranan. Macromolecules 27(21):5937–5942
Karakawa M, Nakatsubo F (2002) An improved method for the preparation of 3-O-benzyl-6-O-pivaloyl-α-d-glucopyranose 1,2,4-orthopivalate. Carbohydr Res 337(10):951–954
Kato T, Yokoyama M, Takahashi A (1978) Melting temperatures of thermally reversible gels. Colloid Polym Sci 256:15–21
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: Fascinating biopolymer and sustainable raw material. Angewandte Chemie-Inter Edn 44(22):3358–3393
Matsumura S, Imai K, Yoshikawa S, Kawada K, Uchibori T (1990) Surface-activities, biodegradability and antimicrobial properties of normal-alkyl glucosides, mannosides and galactosides. J Am Oil Chem Soc 67(12):996–1001
Matsumura S, Kawamura Y, Yoshikawa S, Kawada K, Uchibori T (1993) Surface-activities, biodegradability and antimicrobial properties of glucosamine derivatives containing alkyl chains. J Am Oil Chem Soc 70(1):17–22
Nakatsubo F, Kamitakahara H, Hori M (1996) Cationic ring-opening polymerization of 3,6-di-O-benzyl-α-d-glucose 1,2,4-orthopivalate and the first chemical synthesis of cellulose. J Am Chem Soc 118(7):1677–1681
Nishimura T, Nakatsubo F (1996) First stepwise synthesis of cellulose analogs. Tetrahedron Lett 37(51):9215–9218
Persson B, Nilsson S, Bergman R (1999) Dynamic surface tension of dilute aqueous solutions of nonionic cellulose derivatives in relation to other macromolecular characterization parameters. J Colloid Interface Sci 218(2):433–441
Rees DA (1972) Polysaccharide gels, a molecular view. Chem Ind (London) 19:630–636
Savage AB (1957) Temperature-viscosity relationships for water-soluble cellulose ethers. Ind Eng Chem 49:99
Schmolka IR (1977) Review of block polymer surfactants. J Am Oil Chem Soc 54(3):110–116
Shinoda K, Yamaguchi T, Hori R (1961) The surface tension and the critical micelle concentration in aqueous solution of β-d-alkyl glucosides and their mixtures. Bull Chem Soc Jpn 34(2):237–241
Shinoda K, Yamanaka T, Kinoshita K (1959) Surface chemical properties in aqueous solutions of nonionic surfactants - octyl glycol ether, α-octyl glyceryl ether and octyl glucoside. J Phys Chem 63(5):648–650
Tomecek P, Filgasova M, Horakova V, Machackova A, Lapcik L (2005) Study of surface tension of HEC, CMC and MC water solutions. J Polym Mater 22(1):81–85
Acknowledgements
H. Kamitakahara acknowledges the Alexander von Humboldt Foundation for a research fellowship at University of Jena. We acknowledge Dr. Kazuhisa Hayakawa, Shinetsu Chemical, for providing the MC SM-4. This investigation was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (Nos. 13760132, 15780124, and 18680009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamitakahara, H., Nakatsubo, F. & Klemm, D. New class of carbohydrate-based nonionic surfactants: diblock co-oligomers of tri-O-methylated and unmodified cello-oligosaccharides. Cellulose 14, 513–528 (2007). https://doi.org/10.1007/s10570-007-9128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-007-9128-x