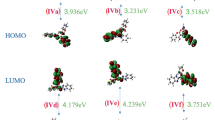
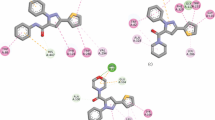
Abstract
In the present study, a new series of ester analogues of substituted coumarin-3-carboxylic acids were synthesized which were typically accessed via a facile esterification reaction between propargyl alcohol and appropriately substituted coumarin-3-carboxylic acids (1–5). This new environmentally benign solid acid catalyst catalyzed, synthetic eco-friendly approach resulted in a noteworthy progress in synthetic efficiency (89–94 % yield), high purity, operational simplicity, mild reaction conditions, cleaner reaction profiles, recyclability of the catalyst and minimizing the production of chemical wastes without using highly toxic reagents for the synthesis. The molecular structure of compound 6 was authenticated by single crystal X-ray crystallographic analysis. The structure and morphology of the catalyst has been established on the basis of FT-IR, scanning electron microscopy–energy dispersion X-ray spectrometry and transmission electron microscopy. The promising bioactive score against enzymatic inhibition prompted us to carry out acetylcholinesterase inhibition screening of the synthesized compounds (6–10). A computer-aided molecular docking study was carried out to validate the specific binding mode of the newly synthesized compounds into the active site of receptor to bear out the specific binding modes of the compounds.
Graphical Abstract















Similar content being viewed by others
References
Ajani OO, Nwinyi OC (2010) J Heterocycl Chem 47:179
Weber US, Steffen B, Siegers CP (1998) Res Commun Mol Pathol Pharmacol 99:193
Patil AD, Freyer AJ, Drake SE, Haltiwanger RC, Bean MF, Taylor PB, Caranfa MJ, Breen AL, Bartus HR, Johnson RK, Hertzberg RP, Westley JW (1993) J Med Chem 36:4131
Yun BS, Lee IK, Ryoo IJ, Yoo ID (2001) J Nat Prod 64:1238
Cheng JF, Ishikawa A, Ono Y, Arrhenius T, Nadzan A (2003) Bioorg Med Chem Lett 13:3647
Zaha AA, Hazem A (2002) Microbiologica 25:213
Backhouse CN, Delporte CL, Negrete RE, Erazo S, Zuniga A, Pinto A, Cassels BK (2001) J Ethnopharmacol 78:27
Tada Y, Shikishima Y, Takaishi Y, Shibata H, Higuti T, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O, Ohmoto Y (2002) Phytochemistry 59:649
Stein AC, Alvarez S, Avancini C, Zacchino S, Poser GV (2006) J Ethnopharmacol 107:95
Whittaker M, Floyd CD, Brown P, Gearing AJH (1999) Chem Rev 99:2735
Maly DJ, Leonetti F, Backes BJ, Dauber DS, Harris JL, Craik CS, Ellman JA (2002) J Org Chem 67:910
Changwong N, Sabphon C, Ingkaninan K, Sawasdee P (2012) Phytother Res 26:392
Piazzi L, Cavalli A, Colizzi F, Belluti F, Bartolini M, Mancini F, Recanatini M, Andrisano V, Rampa A (2008) Bioorg Med Chem Lett 18:423
Garino C, Pietrancosta N, Laras Y, Moret V, Rolland A, Quéléver G, Kraus JL (2006) Bioorg Med Chem Lett 16:1995
Ortega DDS, Murphy BP, Velasquez FJG, Wilson KA, Xie F, Wang Q, Moss MA (2011) Bioorg Med Chem 19:2596
Radić Z, Reiner E, Simeon V (1984) Biochem Pharmacol 33:671
Radić Z, Reiner E, Taylor P (1991) Mol Pharmacol 39:98
Rudolf VS, Kovarik Z, Radić Z, Reiner E (1999) Chem Biol Interact 119–120:119
Pechmann VH, Duisberg C (1884) Chem Ber 17:929
Perkin WH, Henry WS (1875) J Chem Soc 28:10
Brufola G, Fringuelli F, Piermatti O, Pizzo F (1996) Heterocycles 43:1257
Cairns N, Harwood LM, Astles DP (1994) J Chem Soc Perkin Trans 1:3101
Shriner RL (1942) The Reformatsky reaction. Wiley, London, p 1:15
Yavari I, Shoar RH, Zonouzi A (1998) Tetrahedron Lett 39:2391
Al-Zaydi KM (2003) Molecules 8:541
Ghosh PP, Das AR (2012) Tetrahedron Lett 53:3140
Khoobi M, Ramazani A, Foroumadi AR, Hamadi H, Hojjati Z, Shafiee A (2011) J Iran Chem Soc 8:1036
Khurana JM, Kumar S (2009) Tetrahedron Lett 50:4125
Rao P, Konda S, Iqbal J, Oruganti S (2012) Tetrahedron Lett 53:5314
Khan AT, Das DK, Islam K, Das P (2012) Tetrahedron Lett 53:6418
Ray SK, Singh PK, Molleti N, Singh VK (2012) J Org Chem 77:8802
Bagdi AK, Majee A, Hajra A (2013) Tetrahedron Lett 54:3892
Salama TA, Ismail MA, Khalil AGM, Elmorsy SS (2012) ARKIVOC ix:242
Karami B, Khodabakhshi S, Eskandari K (2012) Tetrahedron Lett 53:1445
Jung JC, Lee JH, Oh S, Lee JG, Park OS (2004) Bioorg Med Chem Lett 14:5527
Zhang XS, Li ZW, Shi ZJ (2014) Org Chem Front 1:44
Karimian R, Piri F, Safari AA, Davarpanah SJ (2013) J Nanostruct Chem 3:52
Datta B, Pasha MA (2013) ISRN Org Chem 2013:1
Chavan F, Madje B, Bharad J, Ubale M, Ware M, Shingare M, Shinde N (2008) Bull Catal Soc India 7:41
Gawande MB, Hosseinpour R, Luque R (2013) Curr Org Synth 11:526
Heravi MM, Ajami D, Ghassemzadeh M (1999) Synth Commun 29:1013
Oskooie HA, Heravi MM, Sadnia A, Jannati F, Behbahani FK (2008) Monatsh Chem 139:27
Wu H, Shen Y, Fan LY, Wan Y, Zhang P, Chen CF, Wang WX (2007) Tetrahedron 63:2404
Shaterian HR, Ghashang M, Feyzi M (2008) Appl Catal A Gen 345:128
Baltork IM, Mirkhani V, Moghadam M, Tangestaninejad S, Zolfigol MA, Alibeik MA, Khosropour AR, Kargar H, Hojati SF (2008) Catal Commun 9:894
Zolfigol MA, Veisi H, Mohanazadeh F, Sedrpoushan A (2011) J Heterocycl Chem 48:977
Veisi H (2010) Tetrahedron Lett 51:2109
Shirini F, Zolfigol MA, Salehi P (2006) Curr Org Chem 10:2171
Zolfigol MA (2001) Tetrahedron 57:9509
Gawande MB, Brancoa PS, Varma RS (2013) Chem Soc Rev 42:3371
Gawande MB, Rathi AK, Nogueira ID, Varma RS, Branco PS (2013) Green Chem 15:1895
Bandgar BP, Gawande SS, Muley DB (2010) Green Chem Lett Rev 3:49
Breton GW (1997) J Org Chem 62:8952
Gupta R, Gupta M, Paul S, Gupta R (2009) Bull Korean Chem Soc 30:2419
Hasaninejad A, Zare AK, Sharghi H, Niknam K, Shekouhy M (2007) ARKIVOC xiv:39
Aoyama T, Suzuki T, Nagaoka T, Takido T, Kodomari M (2013) Synth Commun 43:553
Pramitha P, Bahulayan D (2012) Bioorg Med Chem Lett 22:2598
International tables for X-ray crystallography, vol III. Kynoch Press, Birmingham, England (1952)
SAINT, Version 6.02, Bruker AXS, Madison, WI (1999)
XPREP, Version 5.1, Siemens Industrial Automation Inc., Madison, WI (1995)
Sheldrick GM (1997) SHELXL-97: program for crystal structure refinement. University of Göttingen, Göttingen
Ellman GL, Courtney KD, Andres VJ, Feather-Stone RM (1961) Biochem Pharmacol 7:88
Ertl P, Rohde B, Selzer P (2000) J Med Chem 43:3714
Thomsen R, Christensen MH (2006) J Med Chem 11:3315
Schuttelkopf AW, Aalten DMFV (2004) Acta Cryst D60:1355
Mustard D, Ritchie DW (2005) Struct Funct Bioinform 60:269
Discovery Studio v4.0 client Copyright @2005-12 Accelrys software Inc
Hsu KC, Chen YF, Lin SR, Yang JM (2011) BMC Bioinform 12:1
Acknowledgments
Authors thank the Chairman, Department of Chemistry, A.M.U, Aligarh, for providing necessary research facilities, University Sophisticated Instrument Facility (USIF), AMU, Aligarh for providing SEM–EDX facilities, SAIF Panjab University Chandigarh for TEM analysis and spectral studies, Division of Bioscience, Dongguk University, Gyeongju, South Korea is acknowledged for bioassay and X-ray analysis. UGC is also gratefully acknowledged for research fellowship to Faheem Ahmad and Ali Mohammed Malla.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parveen, M., Ahmad, F., Malla, A.M. et al. Catalyst Promoted Synthesis, Computational and Enzyme Inhibition Studies of Coumarin Esters. Catal Lett 144, 2091–2106 (2014). https://doi.org/10.1007/s10562-014-1381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1381-7