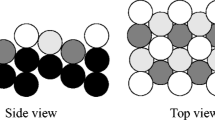
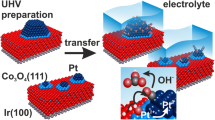
Abstract
Ultrathin manganese oxide films grown on Pt(111) were examined in the low temperature CO oxidation reaction at near atmospheric pressures. Structural characterization was performed by X-ray photoelectron spectroscopy, Auger electron spectroscopy, high-resolution electron energy loss spectroscopy, and temperature programmed desorption. The results show that the reactivity of MnOx ultrathin films is governed by a weakly bonded oxygen species, which may even be formed at low oxygen pressures (~10−6 mbar). For stable catalytic performance at realistic conditions the films required highly oxidizing conditions (CO:O2 < 1:10), otherwise the films dewetted, ultimately resulting in the catalyst deactivation. Comparison with other thin films on Pt(111) shows, that the desorption temperature of weakly bonded oxygen species can be used as a benchmark for its activity in this reaction.
Graphical Abstract












Similar content being viewed by others
References
Freund H-J, Pacchioni G (2008) Oxide ultra-thin films on metals: new materials for the design of supported metal catalysts. Chem Soc Rev 37(10):2224–2242
Giordano L, Pacchioni G (2011) Oxide films at the nanoscale: new structures, new functions, and new materials. Acc Chem Res 44(11):1244–1252
Shaikhutdinov S, Freund H-J (2012) Ultrathin oxide films on metal supports: structure-reactivity relations. Annu Rev Phys Chem 63(1):619–633
Ackermann MD et al (2005) Structure and reactivity of surface oxides on Pt(110) during catalytic CO oxidation. Phys Rev Lett 95(25):255505
Gustafson J et al (2008) Sensitivity of catalysis to surface structure: the example of CO oxidation on Rh under realistic conditions. Phys Rev B 78(4):045423
Lundgren E et al (2006) Surface oxides on close-packed surfaces of late transition metals. J Phys: Condens Matter 18(30):R481
Sun YN et al (2009) Monolayer iron oxide film on platinum promotes low temperature CO oxidation. J Catal 266(2):359–368
Hellman A, Klacar S, Grönbeck H (2009) Low temperature CO oxidation over supported ultrathin MgO films. J Am Chem Soc 131(46):16636–16637
He YB et al (2008) Oxidation of Ir(111): from O–Ir–O trilayer to bulk oxide formation. J Phys Chem C 112(31):11946–11953
Sun Y-N et al (2010) The interplay between structure and CO oxidation catalysis on metal-supported ultrathin oxide films. Angew Chem 49(26):4418–4421
Rogal J, Reuter K, Scheffler M (2008) CO oxidation on Pd(100) at technologically relevant pressure conditions: first-principles kinetic Monte Carlo study. Phys Rev B 77(15):155410
Gustafson J et al (2004) Self-limited growth of a thin oxide layer on Rh(111). Phys Rev Lett 92(12):126102
Flege JI, Hrbek J, Sutter P (2008) Structural imaging of surface oxidation and oxidation catalysis on Ru(0001). Phys Rev B 78(16):165407
Martynova Y et al (2013) CO oxidation over ZnO films on Pt(111) at near-atmospheric pressures. J Catal 301:227–232
Hagendorf C et al (2008) Growth, atomic structure, and vibrational properties of MnO ultrathin films on Pt(111). Phys Rev B 77(7):075406
Sachert S et al (2010) Thickness dependent vibrational and electronic properties of MnO(100) thin films grown on Pt(111). Phys Rev B 81(19):195424
Rizzi GA et al (2001) An X-ray photoelectron diffraction structural characterization of an epitaxial MnO ultrathin film on Pt(111). Surf Sci 482–485(Part 2(0)):1474–1480
Müller F et al (2002) Epitaxial growth of MnO/Ag(001) films. Surf Sci 520(3):158–172
Li F et al (2009) Two-dimensional manganese oxide nanolayers on Pd(100): the surface phase diagram. J Phys: Condens Matter 21(13):134008
Nishimura H et al (2000) Surface structure of MnO/Rh(100) studied by scanning tunneling microscopy and low-energy electron diffraction. AVS, Seattle
Zhang L et al (2012) Growth and vibrational properties of MnOx thin films on Rh(111). Surf Sci 606(19–20):1507–1511
Schnadt J et al (2012) The new ambient-pressure X-ray photoelectron spectroscopy instrument at MAX-lab. J Synchrotron Radiat 19(5):701–704
Feulner P, Menzel D (1980) Simple ways to improve “flash desorption” measurements from single crystal surfaces. J Vac Sci Technol 17(2):662–663
Kostov KL et al (2013) Surface-phonon dispersion of a NiO(100) thin film. Phys Rev B 87(23):235416
Franchini C et al (2006) Density functional study of the polar MnO(111) surface. Phys Rev B 73(15):155402
Martynova Y et al (2012) Low temperature CO oxidation on ruthenium oxide thin films at near-atmospheric pressures. Catal Lett 142(6):657–663
Bagus PS, Ilton ES (2006) Effects of covalency on the p-shell photoemission of transition metals: MnO. Phys Rev B 73(15):155110
Acknowledgments
The Swedish part of the work was supported by the Swedish Research Council (VR) and the Göran Gustafsson Foundation. Prof. J. Schnadt, Dr. J. Knudsen and the MAX-lab staff are gratefully acknowledged for their support. The FHI team acknowledges the support from the COST Action CM1104 “Reducible oxide chemistry, structure and functions”. WW, SS, and SP gratefully acknowledge financial support by the Deutsche Forschungsgemeinschaft through SFB 762 “Functionality of Oxidic Interfaces”. Finally, we thank Prof. L. Spiccia for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martynova, Y., Soldemo, M., Weissenrieder, J. et al. CO Oxidation Over Monolayer Manganese Oxide Films on Pt(111). Catal Lett 143, 1108–1115 (2013). https://doi.org/10.1007/s10562-013-1117-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1117-0