Abstract
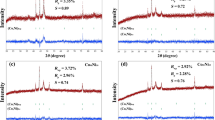
Metal (IV) phosphates of tin, zirconium and titanium were used as catalysts for selective dehydration of sorbitol to isosorbide. Tin phosphate showed the highest selectivity to isosorbide with a moderate conversion. The deactivation rates of catalysts were fitted by an empirical Voorhies equation and the results showed tin phosphate had the lowest deactivation rate with the least values of m and n. Thermal analysis showed deactivation rate was in accordance with coke deposition. The acidity caused by functional groups of the catalysts was the direct factor for catalyst lifetime, which was evidenced by NH3-TPD, XRD, IR and Raman characterizations.







Similar content being viewed by others
References
Blanc B, Bourrel A, Gallezot P, Haas T, Taylor P (2000) Green Chem 2:89
Huber GW, Shabaker JW, Dumesic JA (2003) Science 300:2075
Huber GW, Cortright RD, Dumesic JA (2004) Angew Chem Int Ed 43:1549
Gohil RM (2009) Polym Eng Sci 49:544
Liu AG, Luckett C (2008) US Patent #0249323
LeMaistre JW, Mori TP (1979) US Patent #4169152
Bhatia KK (2002) US Patent #6407266
Hartmann LA (1969) US Patent #3454603
Kurszewska M, Skorupowa E, Madaj J, Konitz A, Wojnowski WA, Winiewski A (2002) Carbohydr Res 337:1261
Qallaf FA, Hodson LF, Johnstone RA, Liu JY, Lu L, Whittaker D (2000) J Mol Catal A: Chem 152:187
Clearfielda A, Thakurb DS (1986) Appl Catal 26:1
Ginestra AL, Patrono P, Berardelli ML, Galli P, Ferragina C, Massucci MA (1987) J Catal 103:346
Marin GB, Beeckman JW, Froment GF (1986) J Catal 97:416
Voorhies A (1945) Ind Eng Chem 37:318
Rostrup-Nielsen JR (1997) Catal Today 37:225
Chen D, Rebo HP, Gronvold A, Moljord K, Holmen A (2000) Microporous Mesoporous Mater 35–36:121
Zhang HB, Zhou KC, Li ZY, Huang SP (2009) J Phys Chem Solids 70:243
Patel SM, Chudasama UV, Ganeshpure PA (2001) Green Chem 3:143
Kim HN, Keller SW, Decher TE (1997) Chem Mater 9:1414
Taher LB, Smiri L, Laligant L, Bail AL (2000) Solid State Sci 2:285
Koleva V, Stefov V, Cahil A, Najdoski M, Soptrajanov B, Engelen B, Lutz HD (2009) J Mol Struct 917:117
Lachgar KA, Tuel A, Brun M, Herrmann JM, Krafft JM, Martin JR, Volta JC, Abon M (1998) J Catal 177:224
Baril M, Assaaoudi H, Butler IS (2005) J Mol Struct 751:168
Acknowledgments
Authors thank the financial support from Post-doctoral Science Fund of China (Grant No. 20070421004) and the Jiangsu Planned Projects for Postdoctoral Research Funds of China (Grant No. 0801018B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, M., Yu, D., Zhang, H. et al. Metal (IV) Phosphates as Solid Catalysts for Selective Dehydration of Sorbitol to Isosorbide. Catal Lett 133, 214–220 (2009). https://doi.org/10.1007/s10562-009-0142-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-0142-5