Abstract
We report on the discovery that paraffins and olefins up to C6 hydrocarbons can be obtained in CO2 electroreduction at room temperature and atmospheric pressure by application of a commercially available Cu-electrode (Eurofysica), provided pretreatment by electropolishing is avoided. The product distribution follows the Schultz–Flory distribution and, depending on the applied potential, the chain growth probability (α) ranges from 0.23 to 0.31, values lower than those obtained in Fischer–Tropsch synthesis over heterogeneous Co- or Fe-based catalysts.
Similar content being viewed by others
1 Introduction
For the conversion of renewable energy into a transportation fuel, many research groups have focused on the production of hydrogen [1]. Besides electrolysis, photocatalytic splitting of water into hydrogen and oxygen has been shown to be feasible [2], even with visible photons [3]. Hydrogen is, however, not a very convenient fuel, with respect to distribution and storage. Besides production of hydrogen, photocatalytic [4] or electrocatalytic conversion of CO2 with H2O is an option [5, 6], yielding fuel-like hydrocarbons which can be exploited in the current infrastructure. Direct electrochemical reduction of CO2 in aqueous solutions has been studied intensively over copper-electrodes, and various reviews on this topic have appeared in the open literature. The product distribution of electro-catalytic CO2 reduction over Cu typically consists of carbon monoxide (CO), methane (CH4), ethene (C2H4), ethanol (C2H5OH) and formic acid (HCOOH) [7–9]. Cu-based electrodes are quite unique in activating CO2, although the Faradaic efficiency is still small: the result of H2O dissociation to H2 [10, 11]. Intensive studies have been conducted to reveal the reaction mechanism of Cu catalyzed CO2 electroreduction, in which a Fischer–Tropsch mechanism, i.e. chain propagation, was proposed as one of the pathways [12, 13]. The group of Hori [14] applied several pre-treatment procedures to alter the surface roughness of Cu electrodes, and concluded that C2H6, a typical product of catalytic Fischer–Tropsch synthesis, was only observed if electro-polishing in acid, typically applied in other studies on CO2 reduction over Cu electrodes, was not conducted. Longer chain hydrocarbons were, however, not reported by the group of Hori for copper electrodes [15, 16]. In the present paper a systematic study of the effect of the electrode pretreatment procedure on the performance is presented, specifically targeting the formation of higher hydrocarbons (C3 +). The result is the exciting discovery that certain Cu-electrodes produce products in CO2 electroreduction with a distribution as typically obtained in the Fischer–Tropsch reaction of syn-gas over heterogeneous Co- or Fe-based catalysts [17]. This is an important scientific discovery, since this is the first tangible evidence that chain propagation occurs during CO2 electroreduction over Cu-electrodes. Furthermore, this work shows that besides on Pt/C-based electrodes [6], a gas to liquid process starting directly from CO2 is potentially also feasible over Cu-electrodes.
2 Experimental
Two commercial Cu foils (Eurofysica Cu foil: 99.9+%, 0.95 mm thick, and Alfa Aesar Cu foil, Puratronic®, 99.9999% (metals basis), 0.25 mm thick) were subsequently polished with 400 and 600 mesh emery papers (3 M 734) for 1 min, and washed with acetone, ethanol, and distilled water. This was followed by ultra-sonication in distilled water for 10 min. The pre-treated Cu-foils were used as electrodes, either with, or without electropolishing in phosphoric acid solution (H3PO4, Aldrich 85 wt.% in water). During the electropolishing procedure, pre-treated Cu-foils were applied as anode, and another Cu foil was used as cathode in one compartment. An absolute potential of 2.3 V was applied between the two Cu electrodes for 1 min [18].
Electrocatalytic conversion of CO2 was carried out in a three-electrode electrochemical cell at ambient temperature and pressure, shown in Fig. 1. A potentiostat, HSV-100 (Hokuto Denko) was used to induce electrocatalytic reduction by applying adequate potential differences over the electrodes. A platinum electrode (Pt, Hokuto Denko, 10 × 20 × 0.1 mm3) was employed as a counter-, and a silver/silver chloride couple (Ag/AgCl, Hokuto Denko, HS205-C) as a reference electrode. The counter electrode was placed in the same compartment as the Cu-electrode (10 cm2). The reference electrode was located in another compartment and a connection to the electrochemical cell was established via a capillary and a salt bridge filled with KCl/agar. A CO2-saturated 0.1 M potassium bicarbonate (KHCO3, Sigma Aldrich ≥ 99.5%) aqueous solution was used as electrolyte. Prior to the electrocatalytic reduction, CO2 was bubbled through for 1 h to make the solution CO2 saturated. After saturation, the pH of the solution was 6.7. The CO2 feed and exit lines were closed off, and the reactor operated in batch mode.
Prior to the electrocatalytic experiments at fixed potential, a cathodic sweep analysis was conducted from the equilibrium electrode potential of the Cu electrode in the solution (−0.050 V vs. Ag/AgCl) to negative electric potential (−2.0 V vs. Ag/AgCl) with 1 mV/s. A slow scan rate was applied to obtain a steady state polarization curve. The starting potential was restricted to the equilibrium potential to avoid the dissolution of Cu ions, which may introduce complications to the analysis, e.g. by re-deposition of Cu ions.
Gas phase hydrocarbons produced by the electrochemical reduction of CO2 at fixed potential were analyzed using a gas chromatograph (GC) equipped with an FID detector (carrier gas: Ar, column: Chrompack Poraplot Q, 50 m × 0.53 mm). Scotty® Scott calibration mixtures (15–20 ppm C1–C4 Hydrocarbons balanced in N2 (UN1956 Class 2–PKG200)), and Scott Speciality Gases mixture C1–C5 Hydrocarbons balanced in N2 (PO Nr. 45319472) were used as calibration gases for hydrocarbons. A volume of 0.5 ml was extracted from the gas volume above the liquid and directly injected on the column for GC analysis. The liquid phase was analyzed for alcohols by a GC equipped with an FID detector (carrier gas: He, column: CP Wax 52 CB, 50 m × 0.32 mm), but these were formed in quantities below the detection, if any. Production of CO was below the detection limit of the TCD applied in the GC analysis (Molecular Sieve 5 Å). Hydrogen was the co-product accounting for the observed Faradaic hydrocarbon efficiency.
The crystal morphology of the electrodes was analyzed by X-ray diffraction (XRD) and elemental analysis was conducted by X-ray Fluorescence Analysis (XRF). XRD measurements of the electrodes were performed using a θ-2 θ diffractometer with an incident beam monochromator and a position sensitive detector (Cu Kα1 radiation), set to an angular range of 30–100° 2θ. XRF measurements were performed on a Panalytical PW2400 wavelength dispersive XRF-spectrometer. The X-ray tube has a Rhodium anode and was operating at 2,400 W.
3 Results and Discussion
The change in current density as a function of cathode potential for the Eurofysica Cu-foil (99.9+%), used without electro-polishing, is shown in Fig. 2. The cathodic current density increases significantly at −0.9 V vs. Ag/AgCl. This increase is due to a combination of water splitting into H2 and O2, and electrocatalytic CO2 conversions. It has been reported that CO and HCOOH are produced at moderate negative electric potential, and hydrocarbons, such as CH4 and C2H4, are produced only at potentials lower than −1.4 V vs. Ag/AgCl, as indicated in Fig. 2 [11]. In the present study we applied an operation window as indicated by the grey area in Fig. 2. In the following the gas phase products formed by reduction of CO2 in this potential range will be discussed in more detail.
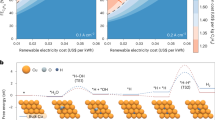
Electro-catalytic CO2 reduction over unpolished Eurofysica Cu-foil results in a unique selectivity. The gas chromatogram of electro-catalytic CO2 reduction at −1.65 V vs. Ag/AgCl (corresponding to galvanostatic operation at 2 mA/cm2), obtained after 720 Coulomb has passed through the cell, is shown in Fig. 3a, and the weight fractions of the detected hydrocarbons are summarized in Fig. 3b. Not only CH4, and C2H4, which are typically reported in CO2 electrocatalytic reduction at −1.65 V vs. Ag/AgCl, but also longer chain paraffins and olefins up to C6 are formed. This is the first experimental evidence that chain propagation is possible in electrocatalytic reduction of CO2 over Cu-electrodes at atmospheric temperature and pressure. It should be noted that a reference experiment in a N2 saturated 0.1 M KHCO3 solution did not result in hydrocarbon production. Therefore, neither bicarbonate (HCO3 −) nor impurities in the system contribute directly to the production of hydrocarbons.
In Fig. 4 this result is presented in a so-called Schultz–Flory product distribution, typically used in Fischer–Tropsch catalysis studies. Chain growth probability is expressed in relation to weight fractions of products as described in Eq. 1 [17],
in which n is the carbon number [−], w n is the weight fraction [−], and a the chain growth probability. The chain growth probability, a, is estimated to be 0.27 from the slope of the trend line shown in Fig. 4. Notably, paraffins show an almost perfect linear correlation, while in the case of olefins C2H4 is an exception. This suggests that C2H4 is produced via another reaction path than chain propagation, resulting in a deviation from the Schultz–Flory distribution. This is in agreement with a previous study using Cu electrodes with CO and CO2 as reactants, in which the reaction paths for C2H4 and CH4 were suggested to be different [19].
Schultz–Flory product distribution of the electrolysis products formed over the 99.9+% Cu (Eurofysica). Galvanostatic operation at 2 mA/cm2 (−1.65 V vs. Ag/AgCl); after passing 720 C through the cell. n: Carbon number [−]; wn: Weight fraction [−]; ○: Paraffins; ●: Olefins; X: Total Cn; - - - -: Trend line for total Cn; ۰-۰-: Trend line only for paraffins (Trend lines are obtained by linear regression)
To further tune the product selectivity of the Eurofysica Cu foil (without electro-polishing), we have studied the effect of the applied current density on the product distribution. Galvanostatic experiments were conducted at different current densities: 1, 5 and 8 mA/cm2. The development of product quantities as a function of time at 5 mA/cm2 is shown in Fig. 5a and the product distribution after 720 C had passed through the cell in Fig. 5b. Since the experiment was conducted in a batch system, the molar concentration increases, rather linearly, as a function of time (the corresponding current passed through the cell is shown at the top axis of Fig. 5a). Deactivation of the electrode, reported in many other studies, is not apparent yet in the first hours of the reaction investigated in the present study. The product selectivity is also rather constant (straight lines in product growth for each of the species); not surprising in view of the low conversion of CO2 in the batch reactor. An induction time is apparent before the products appear. This induction period was also observed in various other experiments in our laboratory. Since the gas phase above the electrolyte was sampled for the analysis, it is likely that the induction period is the result of some solubility of the product gases in the electrolyte. Although these are low, the amount of products formed is as well (μmole range). Increasing the current density from 2 mA/cm2 to 5 mA/cm2 changed the relative product distribution (compare Figs. 3b and 5b), favoring the formation of C2 products, especially ethylene, to the expense of long chain products.
(a) Product development over the 99.9+% Cu (Eurofysica) electrode as a function of reaction time at a current density of 5 mA/cm2. Molar production of C1 and C2 (μmol), left axis. Molar production of C3 and C4 (μmol), right axis. Top axis: Total Electric charge passed through the cell (C). (b) Relative weight fractions of each of the products formed after passing 720 C through the cell
The concentrations of the predominant products (categorized as C1, C2, C3 and C4 hydrocarbons) as a function of current density are shown in Fig. 6. Clearly C2 hydrocarbons maximize at a current density of approximately 5 mA/cm2 (corresponding to a voltage of −1.9 V vs. Ag/AgCl), while the amount of CH4 produced is increased at a current density of 8 mA/cm2. Chain growth probabilities were also calculated for each current density, compared at equal levels of electric charge passed through the cell, i.e. 720 Coulomb. The data are listed in Table 1, together with the corresponding Faradaic efficiencies. Faradaic efficiencies of total hydrocarbon production reach ~7%, when electrolysis was carried out at −1.90 V vs. SHE (5 mA/cm2). Hydrogen was the co-product accounting for the remaining electron consumption (see Fig. 2). In agreement with the trend shown in Fig. 6, the chain growth probability is affected by changing the applied potential. Two phenomena are important to explain the changing chain growth probability and the trend of Fig. 6. Firstly, Hewitt reported that the surface coverage of adsorbed hydrogen increases as a function of increasing negative potential [20]. Second, it has been reported that the CO surface coverage, which is proposed to be a key intermediate in CO2 reduction, is also a function of the applied potential [21]. The coverage of CO was reported to maximize at −1.2 V vs. SHE, while it decreases at more negative potentials [21, 22]. As a consequence of these observations, the surface [H]/[CO or CO2-derived intermediate] ratio is expected to increase at more negative potential. This is equivalent to varying the H2/CO ratio in the “normal” catalytic Fischer–Tropsch reaction at elevated temperatures and pressures over Co or Fe catalysts. Increasing the H2/CO ratio results in increasing total activity, and decreasing chain length [17, 23]. This trend is in agreement with the increasing total activity and decreasing chain growth probability at more negative electric potential observed in this electrocatalytic study. This also explains the increase in relative amount of methane formed at 8 mA/cm2 vs 5 mA/cm2 (Fig. 6).
The Eurofysica Cu foil 99.9+% was also examined after electropolishing. The product distribution changed drastically. Mainly CH4 and C2H4 were formed, with just traces of C2H6 and higher hydrocarbons, as shown in terms of relative weight fractions in Fig. 7a. For this electrode, contrary to what was observed for unpolished electrodes in Fig. 5, the selectivity changes as a function of time, favoring ethane and propane over ethylene (Fig. 7b). Apparently the morphology and/or chemical composition of the electrode surface is changing in the course of the reaction. The most likely change is the formation of oxides, as we will further discuss later.
When the Alfa Aesar high purity foil (99.9999%) was used, with or without electropolishing in concentrated H3PO4, CH4 and C2H4 were the only products formed, in agreement with the literature [7, 10]. Selectivity changes as observed for the polished Eurofysica Cu foil were absent, and C3 + products were never observed. For reasons of brevity, the corresponding curves are not shown. It should be mentioned that applying other transition metal electrodes, besides in the special electrolysis cell configuration of Centi et al. [6], the formation of higher hydrocarbons (C3 +) was only reported at high pressure [24, 25] with efficiencies being extremely low, typically one order of magnitude lower than the hydrocarbon amounts obtained with Cu-electrodes.
The question arises, what is the “magic” ingredient that makes the Eurofysica Cu foil ‘as is’ so uniquely effective. Three explanations can be proposed: (i) the presence of contaminants which are removed by the electropolishing procedure, (ii) a unique surface morphology that is strongly affected by electropolishing, and (iii) the presence of surface oxides, which are removed by electropolishing. To verify the first explanation, elemental analysis of the Alfa Aesar and Eurofysica Cu electrodes was conducted by X-ray Fluorescence Analysis (XRF). From the XRF results, significant differences in the elemental composition between the Eurofysica and the Alfa Aesar electrodes were absent. Furthermore, compositional changes of the Eurofysica Cu electrode before and after polishing could not be established.
To verify the second explanation, the surface exposed index planes of the two types of electrodes were determined with X-ray diffraction (XRD). The XRD patterns reveal that Eurofysica Cu is rather polycrystalline with several crystal indices exposed, i.e. Cu(111), (200), (220) and (311), while Alfa Aesar Cu mainly consists of Cu(200) with a slight contribution of Cu(111), as indicated in Table 2. Although product distributions have been reported to be a function of the exposed crystal plane of Cu-electrodes [9], it is important to note that after electropolishing the X-Ray diffraction pattern of the electrode only shows minor changes, which makes a unique surface morphology being present before electropolishing not very likely.
This leaves the explanation for the remarkable behavior of the Eurofysica electrode to the presence of surface oxides. The polycrystallinity of Eurofysica may facilitate the formation of surface oxide complexes during ambient storage. The pure Alfa Aesar Cu electrodes are single crystalline-like, and, as a consequence, relatively insensitive to oxidation. This explanation is strongly supported by the changing selectivity of the Eurofysica electrode after electropolishing as a function of time. Initially the Eurofysica electrode shows ‘normal’ behavior (comparable to the pure 99.9999% Cu plate), since the electropolishing procedure has removed the surface oxides. In the course of the experiment the electrode is (partially) oxidized in situ as a result of CO2 decomposition, leading to the observed selectivity changes towards Fischer–Tropsch like products. This oxidation, and accompanying selectivity changes are not observed for the Alpha Aesar electrode, presumably because of a higher stability against in situ oxidation as a result of the absence of surface reactive planes. Based on the present experimental data it is not possible to identify the active sites, and the oxidation state of the Cu. The effect of the generation of oxidized active sites on copper electrodes on the product distribution in CO2 reduction has among others previously been investigated by Hori et al. [14] and Momose et al. [26]. Hori and coworkers deliberately oxidized copper electrodes at elevated temperatures in air. It was observed that after severe oxidation, ethane becomes a product of the reaction, whereas methane production decreases. This suggests that indeed surface oxygen would induce ethane formation, the product indicative for chain propagation reactions. Momose states that oxygen present at the electrode surface can lead to an enhanced adsorption of CO2. In analogy to Fischer–Tropsch catalysis, it is thus likely that besides the applied potential, the surface [H]/[CO or CO2-derived intermediate] ratio is decreased as a function of (initial) “oxygen” coverage of the Cu-electrode surface, favoring long chain hydrocarbons.
To further substantiate the role of the oxidation state we have decided to initiate a new study-based on well defined copper electrodes modified in a controlled fashion either by oxidation or by in situ modification as a result of reaction in CO2. Electron microscopy (SEM), XPS, and surface enhanced Raman analyses will be performed to identify morphological changes and changes in the surface oxidation state.
4 Conclusions
In summary, the Eurofysica Cu foil, when not electropolished, converts CO2 into hydrocarbons with a previously never reported Fischer–Tropsch like product distribution. Paraffins and olefins up to C6 were observed. Chain growth probabilities are tunable by altering the applied potential. When the same electrode material was pre-treated by electro-polishing it behaved like a pure Cu electrode: mainly methane and ethene were observed. It is suggested that the oxygen coverage of the electrodes is a function of the surface crystallinity. Surface oxygen is proposed to be an important factor in controlling the selectivity in the CO2 activation.
References
Tzimas E, Catello E, Peteves S (2007) Int J Hydrogen Energy 32:1369
Maeda K, Domen K (2007) J Phys Chem C 111:7851
Maeda K, Teramura K, Lu D, Saito N, Inuoe Y, Domen K (2006) Angew Chemie 45:7806
Anpo M, Yamashita H, Ichihashi Y, Fujii Y, Honda M (1997) J Phys Chem B 101:2632–2636
Electrochemical and Electrocatalytic Reactions of Carbon Dioxide (1993) Elsevier Science Publishers B.V., Amsterdam, The Netherlands
Centi G, Perathoner S, Wine G, Gangeri M (2007) Green Chemistry 9:671
Jitaru M, Lowy DA, Toma M, Toma BC, Oniciu L (1997) J Appl Electrochem 27:875
Chaplin RPS, Wragg AA (2003) J Appl Electrochem 33:1107–1123
Gattrell M, Gupta N, Co A (2006) J Electroanal Chem 594:1
Hori Y, Wakebe H, Tsukamoto T, Koga O (1994) Electrochim Acta 39:1833
Hori Y, Kikuchi K, Murata A, Suzuki S (1986) Chem Letters 897–898
Hori Y, Murata A, Takahashi R (1989) J Chem Soc-Faraday Trans 85:2309
Schwartz M, Vercauteren ME, Sammells AF (1994) J Electrochem Soc 141:3119
Koga O, Nakama K, Murata A, Hori Y (1989) Denki Kagaku 57:1137
Hori Y, Konishi H, Futamura T, Murata A, Koga O, Sakurai H, Oguma K (2005) Electrochim Acta 50:5354
Hori Y, Takahashi I, Koga O, Hoshi N (2003) J Mol Catal A-Chem 199:39
Van der Laan GP, Beenackers AACM (1999) Catal Rev-Sci Eng 41:255
Smith BD, Irish DE, Kedzierzawski P, Augustynski J (1997) J Electrochem Soc 144:4288
Hori Y, Takahashi R, Yoshinami Y, Murata A (1997) J Phys Chem B 101:7075
Hewitt TD, Roy D (1991) Chem Phys Lett 181:407
Gupta N, Gattrell M, MacDougall B (2006) J Appl Electrochem 36:161
Kudo A, Nakagawa S, Tsuneto A, Sakata T (1993) J Electrochem Soc 140:1541
de Deugd RM, Kapteijn F, Moulijn JA (2003) Catal Today 79:495
Ougitani Y, Aizawa T, Sonoyama N, Sakata T (2001) Bull Chem Soc Japan 74:2119
Azuma M, Hashimoto K, Hiramoto M, Watanabe M, Sakata T (1990) J Electrochem Soc 137:1772
Momose Y, Sato K, Ohno O (2002) Surf Interface Anal 34:615
Acknowledgement
Dr. Masahiro Furuya at Central Research Institute of Electric Power Industry (CRIEPI) of Japan, is greatly acknowledged for discussions and for providing the potentiostat. SENECU, the sustainable energy program of the TU Delft, is acknowledged for financial support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Shibata, H., Moulijn, J.A. & Mul, G. Enabling Electrocatalytic Fischer–Tropsch Synthesis from Carbon Dioxide Over Copper-based Electrodes. Catal Lett 123, 186–192 (2008). https://doi.org/10.1007/s10562-008-9488-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9488-3