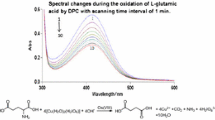
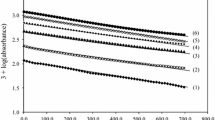
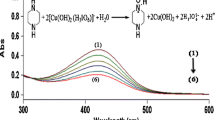
Abstract
The kinetics of osmium(VIII) and ruthenium(III) catalysed oxidation of l-lysine (l-lys) by diperiodatocuprate(III) (DPC) in alkaline medium at a constant ionic strength of 0.15 mol dm−3 was studied spectrophotometrically. The reaction between l-lys and DPC in alkaline medium exhibits 1:2 stoichiometry in both catalysed reaction (l-lys: DPC). The reaction is first order in [DPC] and has less than unit order both in [l-lys] and [alkali]. Increase in periodate concentration decreases the rate. Intervention of free radicals was observed in the reaction. The main products were identified by spot test, IR and GC-MS studies. Probable mechanisms are proposed and discussed. The reaction constants involved in the different steps of the mechanism are calculated. The activation parameters with respect to the slow step of the mechanism are computed and discussed and thermodynamic quantities are also determined. It has been observed that the catalytic efficiency for the present reaction is in the order of Os(VIII) > Ru(III). The active species of catalyst and oxidant have been identified.
Graphical Abstract
The kinetic and mechanistic investigations of the reaction between DPC and l-lysine has been studied in presence of microamounts of ruthenium(III) and osmium(VIII) in alkaline medium. The monoperiodatoargentate(III), [Ru(H2O)5OH]2+ and [OsO4(OH)2]2− are considered as the active species of oxidant, DPC, ruthenium(III) and osmium(VIII) respectively.








Similar content being viewed by others
References
Reddy KB, Sethuram B, Navaneeth Rao T (1984) Indian J Chem 23A:593
Kovat Z (1960) Acta Chim Hung 21:247; Kovat Z (1960) Acta Chim Hung 22:313
Mahadevappa DS, Rangappa KS, Gowda NNM, Thimmegowda B (1986) Int J Chem Kinet 60:589 and references therein
Das AK (2001) Coord Chem Revs 213:307
Agrawal MC, Upadhyay SK (1994) J Sci Ind Res 42:508; Hugar GH, Nandibewoor ST (1994) Transition Met Chem 19:215
Kiran TS, Hiremath DC, Nandibewoor ST (2006) Z Phys Chem 221:501
Harihar AL, Kembhavi MR, Nandibewoor ST (2000) Indian J Chem 39A:769
Saxena OC (1967) Microchem J 12:609
Reddy CS, Vijaya Kumar T (1995) Indian J Chem 34A:615; Kamble DL, Chougale RB, Nandibewoor ST (1996) Indian J Chem 35A:865
Jaiswal PK, Yadava KL (1973) Indian J Chem 11:837; Murthy CP, Sethuram B, Navaneeth Rao T (1981) Z Phys Chem 262:336
Jeffery GH, Bassett J, Mendham J, Denny RC (1996) Vogel’s textbook of quantitative chemical analysis, 5th edn. ELBS, Longman, Essex, p 455
Panigrahi GP, Misro PK (1978) Indian J Chem 16A:201
Vogel AI (1989) A text book of quantitative chemical analysis, 5th edn. ELBS, Longmen Essex, UK, p 371
Kolthoff IM, Meehan EJ, Carr EM (1953) J Am Chem Soc 75:1439; Bhattacharya S, Banerjee P (1996) Bull Chem Soc Japan 69:3475
Moelwyn-Hughes EA (1947) Kinetics of reaction in solutions. Oxford University Press, London, p 297
Reddy KB, Sethuram B, Navaneeth Rao T (1987) Z Phys Chem 268:706
Bailar JC Jr, Emeleus HJ, Nyholm SR, Trotman- Dikenson AF (1975) Comprehensive inorganic chemistry, vol 2. Pergamon press, Oxford, p 1456
Sethuram B (2003) Some aspects of electron transfer reactions involving organic molecules. Allied Publishers, New Delhi, p 78
Reddy KB, Sethuram B, Navaneeth Rao T (1981) Indian J Chem 20A:395; Murthy CP, Sethuram B, Reddy KB, Navaneeth Rao T (1984) Indian J Chem 23A:593
Cotton FA, Wilkinson G (1996) Advanced inorganic chemistry. New York, Wiley Eastern, p 153; Singh HS, Singh RK, Singh SM, Sisodia AK (1977) J Phy Chem 81:1044
Chimatadar SA, Kini AK, Nandibewoor ST (2005) Inorg React Mech 5:231; Desai SM, Halligudi NN, Nandibewoor ST (2002) Transition Met Chem 27:207
Chang R (1981) Physical chemistry with applications to biological systems. Macmillan, New York, p 326
Lister MW (1953) Can J Chem 31:638
Thimmegowda B, Ishwarabhat J (1989) Indian J Chem 28A:43; Bilehal DC, Kulkarni RM, Nandibewoor ST (2003) Z Phys Chem 217:1; Bellakki MB, Mahesh RT, Nandibewoor ST, Jaky M (2005) Proc Nat Acad Sci India 75:177
Kamble DL, Nandibewoor ST (1998) J Phys Org Chem 11:171
Szeverenyi M, Simandi LI (1991) Inorg Chim Acta 186:33; Lott KAK, Symons MCR (1960) Discuss Faraday Soc 29:205; Chougale RB, Hiremath GA, Nandibewoor ST (1997) Polish J Chem 71:1471
Rangappa KS, Raghavendra MP, Mahadevappa DS, Channegouda D (1998) J Org Chem 63:531; Bilehal DC, Kulkarni RM, Nandibewoor ST (2001) Can J Chem 79:1926
Weissberger A, Lewis ES (eds) (1974) Investigations of rates and mechanism of reactions in techniques of chemistry, vol 4. Wiley, New York, p 421
Martinez M, Pitarque MA, Eldik RV (1996) J Chem Soc Dalton Trans 2665; Farokhi SA, Nandibewoor ST (2003) Tetrahedron, 59:7595
Walling C (1957) Free radicals in solution. Academic Press, New York, p 38
Acknowledgments
The authors thank the UGC, New Delhi, for the sanction of research grant (F.30-66/2004(SR) dated 10-11-2004).
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
According to Scheme 2
The total concentration of DPC is given by (Where T and f stands for total and free)
Therefore,
Similarly,
In view of the low concentration of [DPC] and [H3IO 2−6 ] used,
Similarly,
In view of the low concentration of [Ru(III)]) used,
Substituting (II), (III), (IV) and (V) in (I) and omitting the subscripts T and f, we get
Similarly for Os(VIII) catalysis, the rate law can be derived.
Rights and permissions
About this article
Cite this article
Hiremath, D.C., Sirsalmath, K.T. & Nandibewoor, S.T. Osmium(VIII)/Ruthenium(III) Catalysed Oxidation of l-lysine by Diperiodatocuprate(III) in Aqueous Alkaline Medium: A Comparative Mechanistic Approach by Stopped Flow Technique. Catal Lett 122, 144–154 (2008). https://doi.org/10.1007/s10562-007-9361-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9361-9