Abstract
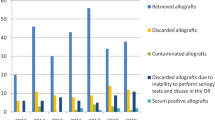
Skeletal muscle and osteoarticular tissue banks are responsible to procure, process, store and distribute tissues, from living and cadaveric donors. The procedures involve the application of protocols covering all aspects of the banking, ensuring the best tissue quality and maximum safety for the recipient. An analysis on the causes of bone tissue discarded by Biotar Tissue Bank between January 2005 and December 2012 was carried. Bone tissue was obtained from both hip and knee replacement (femoral heads and tibial plateau respectively) in living donors treated at different medical–surgical institutions in Argentina. These tissues were processed at the Bank to produce both frozen and lyophilized cancellous bone. Out of 3413 donated bones received by the Bank, 77.55 % resulted in final product, while the remaining 22.44 % was discarded in compliance with the quality standards of both the Bank and the regulatory authority. Comparing the last and the first year of the studied period, the number of discarded tissue increased 3.6 times, while the number of collected bones was approximately 10 times higher. Related to total disposed tissue, reactive serology was the most frequent cause (62.14 %), followed by inappropriate collection/storage of blood sample (30.81 %). A progressive reduction in the percentages of total discard was observed, and this was proportional to inappropriate collection/storage of blood sample. No significant differences were found in the discard rates due to positive serology throughout all the years studied. The success of a tissue bank requires full commitment of all the personnel especially the team members responsible for donor selection and the processing of allografts. It is important to critically screen donors in the early stages of donor recruitment. All of the procedures carried out by the tissue bank are parts of the quality control system which must be strictly carried out. Biotar Tissue Bank is continuously committed to ensure safety to the recipients.




Similar content being viewed by others
References
Abbas G, Bali SL, Abbas N, Dalt DJ (2007) Demand and supply of bone allograft and the role of orthopaedic surgeons. Acta Orthop Belg 73:507–511
Aho AJ, Hirn M, Aro HT et al (1998) Bone bank service in Finland: experience of bacteriologic, serologic and clinical results of the Turku Bone Bank 1972–1995. Acta Orthop Scand 69:559–565
Aponte-Tinao LA, Ritacco LE, Albergo JI et al (2014) The principles and applications of fresh frozen allografts to bone and joint reconstruction. Orthop Clin N Am 45(2):257–269
Aro HT, Aho AJ (1993) Clinical use of bone allografts. Ann Med 25:403–412
Arrington ED, Smith WJ, Chambers HG et al (1996) Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 329:300–309
Asselmeier MA, Caspari RB, Bottenfield S (1993) A review of allograft processing and sterilization techniques and their role in transmission of the human immunodeficiency virus. Am J Sports Med 21:170–175
Ayerza MA, Aponte-Tinao LA, Farfalli GL et al (2009) Joint preservation after extensive curettage of knee giant cell tumors. Clin Orthop Relat Res 467(11):2845–2851
Beckmann NA, Mueller S, Gondan M, Jaeger S, Reiner T, Bitsch RG (2015) Treatment of severe bone defects during revision total knee arthroplasty with structural allografts and porous metal cones—a systematic review. J Arthroplasty 30(2):249–253
Biglione M, Berini C (2013) Aportes y consideraciones sobre la infección por los virus linfotrópicos T humanos tipo 1 y 2 en Argentina. Rev Argent Salud Pública 4(14):32–37
Buck BE, Malinin TI (1994) Human bone and tissue allografts: preparation and safety. Clin Orthop Relat Res 303:8–17
Buck BE, Malinin TI, Brown MD (1989) Bone transplantation and human immunodeficiency virus: an estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res 240:129–136
Busch MP (2004) Should HBV DNA NAT replace HBsAg and/or anti-HBc screening of blood donors? Transfus Clin Biol 11(1):26–32
Calvo R, Figueroa D, Díaz-LedezmaC, Vaisman A, Figueroa F (2011) Bone allografts and the functions of bone banks. Rev Med Chile 139:660–666
Cañadell J, Cornejo F (1987) Banco de hueso de la Clínica Universitaria de Navarra. Rev Med Univ Navarra 31:239–246
Conrad EU, Gretch DR, Obermeyer KR et al (1995) Transmission of the hepatitis-C virus by tissue transplantation. J Bone Joint Surg Am 77(2):214–224
Deijkers RLM, Bloem RM, Petit PLC et al (1997) Contamination of bone allografts analysis of incidence and predisposing factors. J Bone Joint Surg Br 79(1):161–166
Faldini C, Chehrassan M, Miscione MT et al (2011) Single-level anterior cervical discectomy and interbody fusion using PEEK anatomical cervical cage and allograft bone. J Orthop Traumatol 12(4):201–205
Galban E, Benzaken AS (2007) Situación de la sífilis en 20 países de Latinoamerica y el Caribe: año 2006. J bras Doenças Sex Transm 19(3–4):166–172
García Neumayer G (2012) Prevalencia de infecciones transmisibles por transfusión en donantes de sangre de dos instituciones de la ciudad de Rosario. Universidad Abierta Interamericana. Sede Regional Rosario. Facultad de Medicina y Ciencias de la Salud
Humar A, Morris M, Blumberg E et al (2010) Nucleic acid testing (NAT) of organ donors: is the ‘best’ test the right test? A consensus conference report. Am J Transplant 10:889–899
Journeaux SF, Johnson N, Bryce SL et al (1999) Bacterial contamination rates during bone allograft retrieval. J Arthroplasty 14(6):677–681
Lee JH, Wang SI, Choi HR et al (2011) Unicondylar osteoarticular allograft reconstruction of the distal femur in a patient with a traumatic osteoaticular defect of the lateral femoral condyle. Knee Surg Sports Traumatol Arthrosc 19(4):556–558
Mascola L, Kubak B, Radhakrishna S, Mone T, Hunter R, Leiby DA, Kuehnert M, Moore A, Steurer F, Lawrence G, Kun H (2006) Chagas disease after organ transplantation. MMWR 55(29):798–800
Mendicino D, Streiger M, Nepote M et al (2013) Enfermedad de chagas en Santa Fe: situación actual y nuevos desafíos. Comunicaciones del Museo Provincial de Ciencias Naturales “Florentino Ameghino” 17(1):1–23
Muscolo DL, Ayerza MA, Aponte-Tinao LA (2006) Massive allograft use in orthopedic oncology. Orthop Clin N Am 37(1):65–74
Muscolo DL, Ayerza MA, Aponte-Tinao L, Farfalli G (2008) Allograft reconstruction after sarcoma resection in children younger than 10 years old. Clin Orthop Relat Res 466(8):1856–1862
Nather A, David V (2007) Femoral head banking: NUH tissue bank experience. Orthopedics 30:308–312
Palmer SH, Gibbons CLMH, Athanasou NA (1999) The pathology of bone allograft. J Bone Joint Surg Br 81(2):333–335
Raimondo G, Pollicino T, Cacciola I, Squadrito G (2007) Occult hepatitis B virus infection. J Hepatol 46(1):160–170
Raz G, Safir OA, Backstein DJ, Lee PT, Gross AE (2014) Distal femoral fresh osteochondral allografts: follow-up at a mean of twenty-two years. J Bone Joint Surg 96(13):1101–1107
Regis D, Sandri A, Bonetti I et al (2012) A minimum of 10-year follow-up of the Burch–Schneider cage and bulk allografts for the revision of pelvic discontinuity. Arthroplasty 27(6):1057–1063
Romaña C (1961) Epidemiología y distribución geográfica de la enfermedad de Chagas. Bol Oficina Sanit Panam 51:390–403
Sakellariou VI, Babis GC (2014) Management bone loss of the proximal femur in revision hip arthroplasty: update on reconstructive options. World J Orthop 5(5):614–622
Seiler JG 3rd, Johnson J (1999) Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc 9(2):91–97
Simonds RJ (1993) HIV transmission by organ and tissue transplantation. Aids 7:S35–S38
Simonds RJ, Holmberg SD, Hurwitz RL et al (1992) Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med 326(11):726–732
Sninsky JJ, Kwok S (1993) The application of quantitative polymerase chain reaction to therapeutic monitoring. Aids 7:S29–S34
Sugihara S, Van Ginkel AD, Jiya TU et al (1999) Histopathology of retrieved allografts of the femoral head. J Bone Joint Surg Br 81(2):336–341
Tomford WW (2000) Bone allografts: past, present and future. Cell Tissue Bank 1(2):105–109
Tomford WW, Mankin HJ (1999) Bone banking: update on methods and materials. Orthop Clin N Am 30(4):565–570
Tugwell BD, Patel PR, Williams IT et al (2005) Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med 143(9):648–654
Urrutia J, Molina M (2013) Fresh-frozen femoral head allograft as lumbar interbody graft material allows high fusion rate without subsidence. Orthop Traumatol Surg Res 99(4):413–418
Veitch SW, Stroud RM, Toms AD (2010) Compaction bone grafting in tibial plateau fracture fixation. J Trauma 68(4):980–983
Woll JE (2005) Tissue banking overview. Clin Lab Med 25:473–486
Yao F, Seed C, Farrugia A et al (2007) The risk of HIV, HBV, HCV and HTLV infection among musculoskeletal tissue donors in Australia. Am J Transplant 7(12):2723–2726
Yoshikawa A, Gotanda Y, Minegishi K et al (2007) Lengths of hepatitis B viremia and antigenemia in blood donors: preliminary evidence of occult (hepatitis B surface antigen–negative) infection in the acute stage. Transfusion 47(7):1162–1171
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hovanyecz, P., Lorenti, A., Lucero, J.M.J. et al. Living donor bone banking: processing and discarding—from procurement to therapeutic use. Cell Tissue Bank 16, 593–603 (2015). https://doi.org/10.1007/s10561-015-9507-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-015-9507-8