Abstract
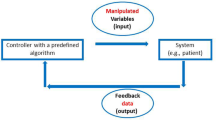
We have developed a novel method for estimating subject-specific hemodynamics during hemorrhage. First, a mathematical model representing a closed-loop circulation and baroreceptor feedback system was parameterized to match the baseline physiology of individual experimental subjects by fitting model results to 1 min of pre-injury data. This automated parameterization process matched pre-injury measurements within 1.4 ± 1.3% SD. Tuned parameters were then used in similar open-loop models to simulate dynamics post-injury. Cardiac output (CO) estimates were obtained continuously using post-injury measurements of arterial blood pressure (ABP) and heart rate (HR) as inputs to the first open-loop model. Secondarily, total blood volume (TBV) estimates were obtained by summing the blood volumes in all the circulatory segments of a second open-loop model that used measured CO as an additional input. We validated the estimation method by comparing model CO results to flowprobe measurements in 14 pigs. Overall, CO estimates had a Bland-Altman bias of −0.30 l/min with upper and lower limits of agreement 0.80 and −1.40 l/min. The negative bias is likely due to overestimation of the peripheral resistance response to hemorrhage. There was no reference measurement of TBV; however, the estimates appeared reasonable and clearly predicted survival versus death during the post-hemorrhage period. Both open-loop models ran in real time on a computer with a 2.4 GHz processor, and their clinical applicability in emergency care scenarios is discussed.





Similar content being viewed by others
References
Altman PL, Dittmer DS, editors. Respiration and circulation. Bethesda, Maryland: Federation of American Societies for Experimental Biology; 1971. 930 pp.
Athanasiades A, Ghorbel F, Clark JW Jr, Niranjan SC, Olansen J, Zwischenberger JB, Bidani A. Energy analysis of a nonlinear model of the normal human lung. J Biol Syst 2000;8:115–39.
Avolio AP. Multi-branched model of the human arterial system. Med Biol Eng Comput 1980;18:709–18.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;8:307–10.
Bourgeois MJ, Gilbert BK, Von Bernuth G, Wood EH. Continuous determination of beat to beat stroke volume from aortic pressure pulses in the dog. Circ Res 1976;39:15–24.
Chan TC, Killeen J, Griswold W, Lenert L. Information technology and emergency medical care during disasters. Acad Emerg Med 2004;11:1229–36.
Cohen SD, Hindmarsh AC. CVODE, a stiff/nonstiff ODE solver in C. Comput Phys 1996;10:138–43.
Dash RK, Bassingthwaighte JB. Blood HbO2 and HbCO2 dissociation curves at varied O2, CO2, pH, 2,3-DPG and temperature levels. Ann Biomed Eng 2004;32:1676–93.
Dash RK, Li Z, Bassingthwaighte JB. Simultaneous blood-tissue exchange of oxygen, carbon dioxide, bicarbonate, and hydrogen ion. Ann Biomed Eng 2006;34:1129–48.
Drzewiecki G, Field S, Moubarak I, Li JK-J. Vessel growth and collapsible pressure-area relationship. Am J Physiol Heart Circ Physiol 1997;273:H2030–43.
Feigl EO. Coronary circulation. In: Patton HD, Fuchs AF, Hille B, Scher AM, Steiner R, editors. Textbook of physiology. Philadelphia, PA: W. B. Saunders; 1989. p. 933–951.
Gao T. Lessons learned from mass casualty drill at Maryland Fire and Rescue Institute. Johns Hopkins University Applied Physics Laboratory: Internal Memo, Laurel, MD: May 11; 2006. p. 1.
Gao T, Greenspan D, Welsh M, Juang RR, Alm A. Vital signs monitoring and patient tracking over a wireless network. In: Proceedings of the 27th Annual International Conference of the IEEE EMBS. p. 1–4, September 2005.
Gao T, Kim MI, White D, Alm AM. Iterative user-centered design of a next generation patient monitoring system for emergency medical response. American Medical Informatics Association 2006 Annual Symposium Proceedings (CD-ROM). p. 284–288, November 2006.
Goedje O, Hoeke K, Lichtwarck-Aschoff M, Faltchauser A, Lamm P, Reichart B. Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: comparison with pulmonary arterial thermodilution. Crit Care Med 1999;27:2407–12.
Hamilton TT, Huber LM, Jessen ME. PulseCO: a less-invasive method to monitor cardiac output from arterial pressure after cardiac surgery. Ann Thorac Surg 2002;74:S1408–12.
Heldt T, Shim EB, Kamm RD, Mark RG. Computational modeling of cardiovascular response to orthostatic stress. J Appl Physiol 2002;92:1239–54.
International Commission on Radiological Protection. Basic anatomical and physiological data for use in radiological protection: reference values. New York: Elsevier Science; 2003. p. 320.
Jansen JRC, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 2001;87:212–22.
Kassab G, Lin DH, Fung Y-CB. Morphometry of pig coronary venous system. Am J Physiol 1994;267(Heart Circ Physiol 36):H2100–13.
Kassab GS, Rider CA, Tang NJ, Fung Y-CB. Morphometry of pig coronary arterial trees. Am J Physiol Heart Circ Physiol 1993;265:H350–65.
Killeen JP, Chan TC, Buono C, Griswold WG, Lenert LA. A wireless first responder handheld device for rapid triage, patient assessment and documentation during mass casualty incidents. American Medical Informatics Association 2006 Annual Symposium Proceedings (CD-ROM). p. 429–433. November 2006.
Klotz S, Hay I, Dickstein ML, Yi G-H, Wang J, Maurer M, Kass DA, Burkhoff D. Single beat estimation of the end-diastolic pressure-volume relationship: a novel method with the potential for noninvasive application. Am J Physiol Heart Circ Physiol 10.1152/ajpheart.01240.2005, January 20, 2006.
Kouchoukos NT, Sheppard LC, McDonald DA. Estimation of stroke volume in the dog by a pulse contour method. Circ Res 1970;26:611–23.
Linton NWF, Linton RAF. Estimation of changes in cardiac output from the arterial blood pressure waveform in the upper limb. Br J Anaesth 2001;86:486–96.
Lu K, Clark JW Jr, Ghorbel FH, Ware DL, Bidani A. A human cardiopulmonary system model applied to the analysis of the Valsalva maneuver. Am J Physiol Heart Circ Physiol 2001;281:H2661–79.
Lu K, Clark JW Jr, Ghorbel FH, Ware DL, Zwischenberger JB, Bidani A. Whole-body gas exchange in human predicted by a cardiopulmonary model. Cardiovasc Eng 2002;3:1–19.
Marino PL. The ICU book. Williams & Wilkins, Baltimore; 1998. p. 928.
Massey T, Gao T, Welsh M, Sharp JH, Sarrafzadeh M. The design of a decentralized electronic triage system. American Medical Informatics Association 2006 Annual Symposium Proceedings (CD-ROM). p. 544–548, November 2006.
McCombie DB, Reisner AT, Asada HH. Laguerre-model blind system identification: cardiovascular dynamics estimated from multiple peripheral circulatory signals. IEEE Trans Biomed Eng 2005;52:1889–901.
Milnor WR. Hemodynamics. Baltimore: Williams and Wilkins; 1982. p. 390.
Mohrman DE, Heller LJ. Cardiovascular physiology. 5th ed. New York McGraw-Hill; 2003. p. 257.
Nelder JA, Mead R. A simplex method for function minimization. Comput J 1965;7:308–13.
Olansen JB, Clark JW, Khoury D, Ghorbel F, Bidani A. A closed-loop model of the canine cardiovascular system that includes ventricular interaction. Comp Biomed Res 2000;33:260–95.
Redling JD, Akay M. Noninvasive cardiac output estimation: a preliminary study. Biol Cybern 1997;77:111–22.
Rideout VC. Mathematical computer modeling of physiological systems. Englewood Cliffs, NJ: Prentice Hall; 1991. p . 261.
Rödig G, Prasser C, Keyl C, Liebold A, Hobbhahn J. Continuous cardiac output measurement: pulse contour analysis vs. thermodilution technique in cardiac surgical patients. Brit J Anaesth 1999;82:525–30.
Romano SM, Pistolesi M. Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med 2002;30:1834–41.
Rosse C, Gaddum-Rosse P. Hollinshead’s textbook of anatomy. 5th ed. Lippincott-Raven, Philadelphia; 1997. p. 902.
Saeed M, Mark RG. Multiparameter trend monitoring and intelligent displays using wavelet analysis. Comp Cardiol 2000;27:797–800.
Sagawa K, Lie RK, Schaefer J. Translation of Otto Frank’s Paper “Die Grundform des Arteriellen Pulses” Zeitschrift für Biologie 37: 483–526 (1899). J Mol Cell Cardiol 1990;22:253–77.
Scher AM. Events of the cardiac cycle: measurements of pressure, flow and volume. In: Patton HD, Fuchs AF, Hille B, Scher AM, Steiner R, editors. Textbook of physiology, vol. 2. Philadelphia: W. B. Saunders; 1989. p. 834–47.
Scoletta S, Romano SM, Biagioli B, Capannini G, Giomarelli P. Pressure recording analytical method (PRAM) for measurement of cardiac output during various haemodynamic states. Br J Anaeth 2005;95:159–65.
Spickler JW, Kezdi P, Geller E. Transfer characteristics of the carotid sinus pressure control system. In: Kezdi P, editor. Baroreceptors and hypertension. Dayton: Pergamon; 1967. p. 31–40.
Sun Y, Beshara M, Lucariello RJ, and Chiaramida SA. A comprehensive model for right-left heart interaction under the influence of pericardium and baroreflex. Am J Physiol Heart Circ Physiol 1997;272:H1499–515.
Toyota E, Fujimoto K, Ogasawara Y, Kajita T, Shigeto F, Matsumoto T, Goto M, Kajiya F. Dynamic changes in three-dimensional architecture and vascular volume of transmural coronary microvasculature between diastolic- and systolic-arrested rat hearts. Circulation 2002;105:621–6
Warner HR, Swan HJC, Connolly DC, Tompkins RG, Wood EH. Quantitation of beat-to-beat changes in stroke volume from the aortic pulse contour in man. J Appl Physiol 1953;5:495–507.
Wesseling KH, Jansen JRC, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 1993;74:2566–73
Wesseling KH, Settels JJ. Circulatory model of baro- and cardio-pulmonary reflexes. In: Di Rienzo M, editor. Blood pressure and heart rate variability. IOS Press; 1992. p. 56–67.
Zinemanas D, Beyar R, Sideman S. Relating mechanics, blood flow and mass transport in the cardiac muscle. Int J Heat Mass Trans 1994;37(Suppl. 1):191–205.
Acknowledgements
This study was supported by DARPA grant #W81XWH0420012 and NSF grant BES-0506477. Capt. Eric Ansorge and Lt. Col. Mac Fudge (Institute of Surgical Research, Fort Sam Houston) provided experimental data, and Jim Rees (University of Michigan) produced beat-by-beat heart rate data files from the ECG recordings. Kay Sterner assisted with manuscript preparation, and Erik Butterworth and Gary Raymond provided software support. The CO estimation model is available at http://physiome.org/redirect/coerh.html.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Equations of the Open-loop Cardiac Output Estimation Model
Lower-case subscript key:
-
0: unstressed volume
-
a: left or right atrium
-
d: diastolic
-
i: left atrium, left ventricle, right atrium or right ventricle
-
j: circulatory segment immediately downstream of the i segment
-
s: systolic
-
v: left or right ventricle
Four-chamber Heart
Heart chamber activation function
Discrete functions to set heart beat start time and heart period
In the model the x domain was created as the set of all positive integers, and used to access R wave event times and heart periods indexed by heartbeat number in an external data file created from empirical ECG data. The functions m and n increased by 1 after each heart cycle completed, and t HB was set to the R wave event time for each heartbeat. The heart period HP was also set discretely for each indexed heartbeat.
for \((t\ge t_{\rm HB}(n+1) - \hbox{PR}_{\rm INT} - offv)\{\)
for \((t\ge t_{\rm HB}(m+1) - offv)\{\)
Heart chamber pressure-volume relationships
Heart flows from the ith to jth chamber
Systemic Circulation
Aortic flow estimate
Smoothed pulmonary valve flow
Cardiac output estimate
Stroke volume
Time-shifted, measured arterial blood pressure
Approximate “derivative” of arterial blood pressure, produced using a lag operator with a small time constant
Tuned scaling factor for systemic veins PV relationship
Aortic afterload set by time-shifted arterial blood pressure data
Systemic circulation pressures
Ψ is given by \(\Uppsi=K_{\rm XP}/(e^{v/k_{XV}} - 1)\) where V is volume and K XP and K XV are curve-shaping constants (see Table 3).
Systemic circulation forward flow
Systemic circulation radial flow
Nonlinear systemic resistances
Pulmonary Circulation
Pulmonary circulation pressures
Pulmonary circulation forward flows
Pulmonary circulation radial flows
Coronary Circulation
Coronary circulation pressures
Coronary circulation flows
Baroreceptor
Transfer function for carotid sinus firing frequency
Equations of efferent pathways
Blood Volumes
Total blood volume
Blood volume in heart
Blood volume in coronary circulation
Blood volume in systemic arterial system
Blood volume in systemic venous system
Blood volume in pulmonary arterial system
Rights and permissions
About this article
Cite this article
Neal, M.L., Bassingthwaighte, J.B. Subject-specific Model Estimation of Cardiac Output and Blood Volume During Hemorrhage. Cardiovasc Eng 7, 97–120 (2007). https://doi.org/10.1007/s10558-007-9035-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10558-007-9035-7