Abstract
Purpose
Arterial stiffness is an inevitable consequence of the aging process and is considered an early stage in the development of cardiovascular diseases. The perivascular adipose tissue (PVAT) is a distinct functional integral layer of the vasculature actively involved in blood pressure regulation and atherosclerosis development via PVAT-derived paracrine/autocrine factors. However, there is little knowledge regarding the relationship between PVAT and arterial stiffness.
Methods
Using unique mice lacking PVAT, high-fat diet-induced obesity, and in mice overexpressing brown adipocyte selective mitoNEET, we investigated the relationship between PVAT and arterial stiffness in mice.
Results
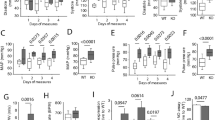
We found that lack of PVAT enhanced arterial stiffness in aging mice. High-fat diet feeding of aging C57BL/6J wild-type mice significantly induced hypertrophic PVAT and enhanced arterial stiffness. Furthermore, the expression of mitoNEET, a mitochondrial membrane protein related to energy expenditure, was significantly increased by pioglitazone treatment, while reduced in the hypertrophic PVAT induced by high-fat diet. Overexpression of mitoNEET in PVAT reduced the expression of inflammatory genes and was associated with lower pulse wave velocity in aging mice.
Conclusions
These data indicate that local PVAT homeostasis especially inflammation in PVAT is associated with arterial stiffness development. Pioglitazone-induced mitoNEET in PVAT prevents PVAT inflammation and is negatively associated with arterial stiffness. These findings provide new experimental insight into the roles of pioglitazone on PVAT in arterial stiffness and indicate that PVAT might be a target to treat or prevent cardiovascular disease.






Similar content being viewed by others
References
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47.
Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–9.
Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49(6):1202–6.
O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46(1):200–4.
McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol. 2007;34(7):665–71.
Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012;5(3):264–73.
Kelly R, Hayward C, Avolio A, O’Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80(6):1652–9.
Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann N Y Acad Sci. 2007;1100:353–60.
Osborne-Pellegrin M, Labat C, Mercier N, Challande P, Lacolley P. Changes in aortic stiffness related to elastic fiber network anomalies in the Brown Norway rat during maturation and aging. Am J Physiol Heart Circ Physiol. 2010;299(1):H144–52.
Chang L, Milton H, Eitzman DT, Chen YE. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J. 2012;77(1):11–8.
Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126(9):1067–78.
Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124(10):1160–71.
Seifalian AM, Filippatos TD, Joshi J, Mikhailidis DP. Obesity and arterial compliance alterations. Curr Vasc Pharmacol. 2010;8(2):155–68.
Selcuk A, Bulucu F, Kalafat F, Cakar M, Demirbas S, Karaman M, et al. Skinfold thickness as a predictor of arterial stiffness: obesity and fatness linked to higher stiffness measurements in hypertensive patients. Clin Exp Hypertens. 2013;35(6):459–64.
Thanassoulis G, Massaro JM, Corsini E, Rogers I, Schlett CL, Meigs JB, et al. Periaortic adipose tissue and aortic dimensions in the Framingham heart study. J Am Heart Assoc. 2012;1(6):e000885.
Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18(10):1539–49.
Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci U S A. 2007;104(13):5318–23.
Harashima K, Hayashi J, Miwa T, Tsunoda T. Long-term pioglitazone therapy improves arterial stiffness in patients with type 2 diabetes mellitus. Metabolism. 2009;58(6):739–45.
Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, et al. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286(2):E252–60.
Xiong W, Zhao X, Garcia-Barrio MT, Zhang J, Lin J, Chen YE, et al. MitoNEET in perivascular adipose tissue blunts atherosclerosis under mild cold condition in mice. Front Physiol. 2017;8:1032.
Kusminski CM, Park J, Scherer PE. MitoNEET-mediated effects on browning of white adipose tissue. Nat Commun. 2014;5:3962.
Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, et al. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation. 2009;119(16):2161–9.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605.
Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009;297(5):E999–E1003.
Pirro M, Schillaci G, Savarese G, Gemelli F, Vaudo G, Siepi D, et al. Low-grade systemic inflammation impairs arterial stiffness in newly diagnosed hypercholesterolaemia. Eur J Clin Investig. 2004;34(5):335–41.
Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–43.
Boden G, Cheung P, Mozzoli M, Fried SK. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism. 2003;52(6):753–9.
Hallakou S, Doare L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, et al. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46(9):1393–9.
Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101(6):1354–61.
Kiyici S, Ersoy C, Kaderli A, Fazlioglu M, Budak F, Duran C, et al. Effect of rosiglitazone, metformin and medical nutrition treatment on arterial stiffness, serum MMP-9 and MCP-1 levels in drug naive type 2 diabetic patients. Diabetes Res Clin Pract. 2009;86(1):44–50.
Yu J, Jin N, Wang G, Zhang F, Mao J, Wang X. Peroxisome proliferator-activated receptor gamma agonist improves arterial stiffness in patients with type 2 diabetes mellitus and coronary artery disease. Metabolism. 2007;56(10):1396–401.
Ryan KE, McCance DR, Powell L, McMahon R, Trimble ER. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis. 2007;194(2):e123–30.
Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52.
Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci. 2010;1212:E20–36.
Kim S, Moustaid-Moussa N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr. 2000;130(12):3110S–5S.
Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev. 1969;49(2):330–425.
De Schutter A, Lavie CJ, Patel DA, Artham SM, Milani RV. Relation of body fat categories by Gallagher classification and by continuous variables to mortality in patients with coronary heart disease. Am J Cardiol. 2013;111(5):657–60.
Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110(1):77–82.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76.
Calabro P, Yeh ET. Obesity, inflammation, and vascular disease: the role of the adipose tissue as an endocrine organ. Subcell Biochem. 2007;42:63–91.
Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis. 2012;225(1):99–104.
Shields KJ, Stolz D, Watkins SC, Ahearn JM. Complement proteins C3 and C4 bind to collagen and elastin in the vascular wall: a potential role in vascular stiffness and atherosclerosis. Clin Transl Sci. 2011;4(3):146–52.
Chen JY, Tsai PJ, Tai HC, Tsai RL, Chang YT, Wang MC, et al. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol. 2013;33(4):839–46.
Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, et al. Bmal1 in perivascular adipose tissue regulates resting phase blood pressure through transcriptional regulation of angiotensinogen. Circulation. 2018;138:67–79.
Hamblin M, Chang L, Zhang H, Yang K, Zhang J, Chen YE. Vascular smooth muscle cell peroxisome proliferator-activated receptor-gamma mediates pioglitazone-reduced vascular lesion formation. Arterioscler Thromb Vasc Biol. 2011;31(2):352–9.
Hamblin M, Chang L, Zhang H, Yang K, Zhang J, Chen YE. Vascular smooth muscle cell peroxisome proliferator-activated receptor-gamma deletion promotes abdominal aortic aneurysms. J Vasc Surg. 2010;52(4):984–93.
Vernay A, Marchetti A, Sabra A, Jauslin TN, Rosselin M, Scherer PE, et al. MitoNEET-dependent formation of intermitochondrial junctions. Proc Natl Acad Sci U S A. 2017;114(31):8277–82.
Funding
This work was supported by NIH grants HL122664 (to L. Chang) and HL088391 (to Y.E. Chen).
Author information
Authors and Affiliations
Contributions
LC designed the studies, LC and XZ performed the experiments, and JZ generated mitoNEET-Tg mice. LC and YC analyzed the data; LC and MGB wrote the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants and/or specimens.
Rights and permissions
About this article
Cite this article
Chang, L., Zhao, X., Garcia-Barrio, M. et al. MitoNEET in Perivascular Adipose Tissue Prevents Arterial Stiffness in Aging Mice. Cardiovasc Drugs Ther 32, 531–539 (2018). https://doi.org/10.1007/s10557-018-6809-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-018-6809-7