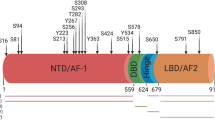
Abstract
Knowledge of the molecular events that contribute to prostate cancer progression has created opportunities to develop novel therapy strategies. It is now well established that c-Src, a non-receptor tyrosine kinase, regulates a complex signaling network that drives the development of castrate-resistance and bone metastases, events that signal the lethal phenotype of advanced disease. Preclinical studies have established a role for c-Src and Src Family Kinases (SFKs) in proliferation, angiogenesis, invasion and bone metabolism, thus implicating Src signaling in both epithelial and stromal mechanisms of disease progression. A number of small molecule inhibitors of SFK now exist, many of which have demonstrated efficacy in preclinical models and several that have been tested in patients with metastatic castrate-resistant prostate cancer. These agents have demonstrated provocative clinic activity, particularly in modulating the bone microenvironment in a therapeutically favorable manner. Here, we review the discovery and basic biology of c-Src and further discuss the role of SFK inhibitors in the treatment of advanced prostate cancer.


Similar content being viewed by others
References
Rous, P. (1911). A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med, 13, 397–411.
Martin, G. S. (1970). Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature, 227, 1021–1023.
Stehelin, D., Varmus, H. E., Bishop, J. M., & Vogt, P. K. (1976). DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature, 260, 170–173.
Collett, M. S., & Erikson, R. L. (1978). Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A, 75, 2021–2024.
Hunter, T., & Sefton, B. M. (1980). Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A, 77, 1311–1315.
Martin, G. S. (2001). The hunting of the Src. Nat Rev Mol Cell Biol, 2, 467–475.
Thomas, S. M., & Brugge, J. S. (1997). Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol, 13, 513–609.
Sicheri, F., & Kuriyan, J. (1997). Structures of Src-family tyrosine kinases. Curr Opin Struct Biol, 7, 777–785.
Patwardhan, P., & Resh, M. D. (2010). Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol Cell Biol, 30, 4094–4107.
Alland, L., Peseckis, S. M., Atherton, R. E., Berthiaume, L., & Resh, M. D. (1994). Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem, 269, 16701–16705.
Stahl, M. L., Ferenz, C. R., Kelleher, K. L., Kriz, R. W., & Knopf, J. L. (1988). Sequence similarity of phospholipase C with the non-catalytic region of src. Nature, 332, 269–272.
Cohen, G. B., Ren, R., & Baltimore, D. (1995). Modular binding domains in signal transduction proteins. Cell, 80, 237–248.
Moarefi, I., LaFevre-Bernt, M., Sicheri, F., et al. (1997). Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature, 385, 650–653.
Moran, M. F., Koch, C. A., Anderson, D., et al. (1990). Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc Natl Acad Sci U S A, 87, 8622–8626.
Hanks, S. K., Quinn, A. M., & Hunter, T. (1988). The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science, 241, 42–52.
Ingley, E. (2008). Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta, 1784, 56–65.
Cooper, J. A., Gould, K. L., Cartwright, C. A., & Hunter, T. (1986). Tyr527 is phosphorylated in pp 60c-src: implications for regulation. Science, 231, 1431–1434.
Kmiecik, T. E., & Shalloway, D. (1987). Activation and suppression of pp 60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell, 49, 65–73.
Xu, W., Harrison, S. C., & Eck, M. J. (1997). Three-dimensional structure of the tyrosine kinase c-Src. Nature, 385, 595–602.
Okada, M., Nada, S., Yamanashi, Y., Yamamoto, T., & Nakagawa, H. (1991). CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem, 266, 24249–24252.
Chong, Y. P., Mulhern, T. D., & Cheng, H. C. (2005). C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)—endogenous negative regulators of Src-family protein kinases. Growth Factors, 23, 233–244.
Kim, L. C., Song, L., & Haura, E. B. (2009). Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol, 6, 587–595.
Irby, R. B., Mao, W., Coppola, D., et al. (1999). Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet, 21, 187–190.
Belsches, A. P., Haskell, M. D., & Parsons, S. J. (1997). Role of c-Src tyrosine kinase in EGF-induced mitogenesis. Front Biosci, 2, d501–d518.
Parsons, J. T., & Parsons, S. J. (1997). Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol, 9, 187–192.
Krystal, G. W., DeBerry, C. S., Linnekin, D., & Litz, J. (1998). Lck associates with and is activated by Kit in a small cell lung cancer cell line: inhibition of SCF-mediated growth by the Src family kinase inhibitor PP1. Cancer Res, 58, 4660–4666.
Biscardi, J. S., Tice, D. A., & Parsons, S. J. (1999). c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res, 76, 61–119.
Abram, C. L., & Courtneidge, S. A. (2000). Src family tyrosine kinases and growth factor signaling. Exp Cell Res, 254, 1–13.
Dey, A., She, H., Kim, L., et al. (2000). Colony-stimulating factor-1 receptor utilizes multiple signaling pathways to induce cyclin D2 expression. Mol Biol Cell, 11, 3835–3848.
Hong, L., Munugalavadla, V., & Kapur, R. (2004). c-Kit-mediated overlapping and unique functional and biochemical outcomes via diverse signaling pathways. Mol Cell Biol, 24, 1401–1410.
Mohamed, A. S., Rivas-Plata, K. A., Kraas, J. R., Saleh, S. M., & Swope, S. L. (2001). Src-class kinases act within the agrin/MuSK pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring, and clustering. J Neurosci, 21, 3806–3818.
Maejima, Y., Ueba, H., Kuroki, M., et al. (2003). Src family kinases and nitric oxide production are required for hepatocyte growth factor-stimulated endothelial cell growth. Atherosclerosis, 167, 89–95.
Bromann, P. A., Korkaya, H., & Courtneidge, S. A. (2004). The interplay between Src family kinases and receptor tyrosine kinases. Oncogene, 23, 7957–7968.
Fizazi, K. (2007). The role of Src in prostate cancer. Ann Oncol, 18, 1765–1773.
Goldenberg-Furmanov, M., Stein, I., Pikarsky, E., et al. (2004). Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res, 64, 1058–1066.
Posadas, E. M., Al-Ahmadie, H., Robinson, V. L., et al. (2009). FYN is overexpressed in human prostate cancer. BJU Int, 103, 171–177.
Tatarov, O., Mitchell, T. J., Seywright, M., Leung, H. Y., Brunton, V. G., & Edwards, J. (2009). SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res, 15, 3540–3549.
Cai, H., Smith, D. A., Memarzadeh, S., Lowell, C. A., Cooper, J. A., & Witte, O. N. (2011). Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci U S A, 108, 6579–6584.
Cai, H., Babic, I., Wei, X., Huang, J., & Witte, O. N. (2011). Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res, 71, 862–872.
Park, S. I., Zhang, J., Phillips, K. A., et al. (2008). Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res, 68, 3323–3333.
Chang, Y. M., Bai, L., Liu, S., Yang, J. C., Kung, H. J., & Evans, C. P. (2008). Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene, 27, 6365–6375.
Gray, M. J., Zhang, J., Ellis, L. M., et al. (2005). HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene, 24, 3110–3120.
Niu, G., Wright, K. L., Huang, M., et al. (2002). Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene, 21, 2000–2008.
Trevino, J. G., Summy, J. M., Gray, M. J., et al. (2005). Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res, 65, 7214–7222.
Karantanos, T., Corn, P. G., Thompson, T. C. (2013). Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene
Migliaccio, A., Castoria, G., Di, D. M., et al. (2000). Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J, 19, 5406–5417.
Migliaccio, A., Castoria, G., Di, D. M., et al. (2002). Sex steroid hormones act as growth factors. J Steroid Biochem Mol Biol, 83, 31–35.
Zhoul, J., Hernandez, G., Tu, S. W., Huang, C. L., Tseng, C. P., & Hsieh, J. T. (2005). The role of DOC-2/DAB2 in modulating androgen receptor-mediated cell growth via the nongenomic c-Src-mediated pathway in normal prostatic epithelium and cancer. Cancer Res, 65, 9906–9913.
Scher, H. I., & Sawyers, C. L. (2005). Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol, 23, 8253–8261.
Chen, Y., Clegg, N. J., & Scher, H. I. (2009). Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol, 10, 981–991.
Guo, Z., Dai, B., Jiang, T., et al. (2006). Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell, 10, 309–319.
Asim, M., Siddiqui, I. A., Hafeez, B. B., Baniahmad, A., & Mukhtar, H. (2008). Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene, 27, 3596–3604.
Lee, L. F., Guan, J., Qiu, Y., & Kung, H. J. (2001). Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol, 21, 8385–8397.
Desai, S. J., Ma, A. H., Tepper, C. G., Chen, H. W., & Kung, H. J. (2006). Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res, 66, 10449–10459.
Hobisch, A., Eder, I. E., Putz, T., et al. (1998). Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res, 58, 4640–4645.
Lee, L. F., Louie, M. C., Desai, S. J., et al. (2004). Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene, 23, 2197–2205.
Ding, Y., Wang, X., Xu, A., et al. (2011). Associations of saposin C, Src, and androgen receptor upregulate the expression and function of androgen receptor in human prostate cancer cells. J Cell Biochem, 112, 818–828.
Asim, M., Hafeez, B. B., Siddiqui, I. A., et al. (2011). Ligand-dependent corepressor acts as a novel androgen receptor corepressor, inhibits prostate cancer growth, and is functionally inactivated by the Src protein kinase. J Biol Chem, 286, 37108–37117.
Liu, Y., Karaca, M., Zhang, Z., Gioeli, D., Earp, H. S., & Whang, Y. E. (2010). Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene, 29, 3208–3216.
Migliaccio, A., Varricchio, L., de Falco, A., et al. (2007). Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene, 26, 6619–6629.
Zhu, D., & Bourguignon, L. Y. (1998). The ankyrin-binding domain of CD44s is involved in regulating hyaluronic acid-mediated functions and prostate tumor cell transformation. Cell Motil Cytoskeleton, 39, 209–222.
Wu, D., Thakore, C. U., Wescott, G. G., McCubrey, J. A., & Terrian, D. M. (2004). Integrin signaling links protein kinase Cepsilon to the protein kinase B/Akt survival pathway in recurrent prostate cancer cells. Oncogene, 23, 8659–8672.
Mitra, S. K., & Schlaepfer, D. D. (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol, 18, 516–523.
Ren, J., Bharti, A., Raina, D., Chen, W., Ahmad, R., & Kufe, D. (2006). MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene, 25, 20–31.
Parri, M., Buricchi, F., Giannoni, E., et al. (2007). EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem, 282, 19619–19628.
Sabbota, A. L., Kim, H. R., Zhe, X., Fridman, R., Bonfil, R. D., & Cher, M. L. (2010). Shedding of RANKL by tumor-associated MT1-MMP activates Src-dependent prostate cancer cell migration. Cancer Res, 70, 5558–5566.
Varkaris, A., Gaur, S., Parikh, N. U., et al. (2013). Ligand-independent activation of MET through IGF-1/IGF-1R signaling. Int J Cancer, 133, 1536–1546.
Yu, H. S., Lin, T. H., & Tang, C. H. (2013). Bradykinin enhances cell migration in human prostate cancer cells through B2 receptor/PKCdelta/c-Src dependent signaling pathway. Prostate, 73, 89–100.
Giannoni, E., Buricchi, F., Raugei, G., Ramponi, G., & Chiarugi, P. (2005). Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol, 25, 6391–6403.
Destaing, O., Block, M. R., Planus, E., & Albiges-Rizo, C. (2011). Invadosome regulation by adhesion signaling. Curr Opin Cell Biol, 23, 597–606.
Wadhawan, A., Smith, C., Nicholson, R. I., Barrett-Lee, P., & Hiscox, S. (2011). Src-mediated regulation of homotypic cell adhesion: implications for cancer progression and opportunities for therapeutic intervention. Cancer Treat Rev, 37, 234–241.
Burridge, K., & Chrzanowska-Wodnicka, M. (1996). Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol, 12, 463–518.
Westhoff, M. A., Serrels, B., Fincham, V. J., Frame, M. C., & Carragher, N. O. (2004). SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol, 24, 8113–8133.
Webb, D. J., Donais, K., Whitmore, L. A., et al. (2004). FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol, 6, 154–161.
Harris, T. J., & Tepass, U. (2010). Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol, 11, 502–514.
Behrens, J., Vakaet, L., Friis, R., et al. (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol, 120, 757–766.
Hamaguchi, M., Matsuyoshi, N., Ohnishi, Y., Gotoh, B., Takeichi, M., & Nagai, Y. (1993). p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J, 12, 307–314.
Mariner, D. J., Anastasiadis, P., Keilhack, H., Bohmer, F. D., Wang, J., & Reynolds, A. B. (2001). Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem, 276, 28006–28013.
Woodcock, S. A., Rooney, C., Liontos, M., et al. (2009). SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol Cell, 33, 639–653.
Kelley, L. C., Ammer, A. G., Hayes, K. E., et al. (2010). Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J Cell Sci, 123, 3923–3932.
Boateng, L. R., & Huttenlocher, A. (2012). Spatiotemporal regulation of Src and its substrates at invadosomes. Eur J Cell Biol, 91, 878–888.
Park, S. J., Suetsugu, S., & Takenawa, T. (2005). Interaction of HSP90 to N-WASP leads to activation and protection from proteasome-dependent degradation. EMBO J, 24, 1557–1570.
Seals, D. F., Azucena, E. F., Jr., Pass, I., et al. (2005). The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell, 7, 155–165.
Buschman, M. D., Bromann, P. A., Cejudo-Martin, P., Wen, F., Pass, I., & Courtneidge, S. A. (2009). The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell, 20, 1302–1311.
Summy, J. M., & Gallick, G. E. (2003). Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev, 22, 337–358.
Nam, S., Kim, D., Cheng, J. Q., et al. (2005). Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res, 65, 9185–9189.
Rabbani, S. A., Valentino, M. L., Arakelian, A., Ali, S., & Boschelli, F. (2010). SKI-606 (Bosutinib) blocks prostate cancer invasion, growth, and metastasis in vitro and in vivo through regulation of genes involved in cancer growth and skeletal metastasis. Mol Cancer Ther, 9, 1147–1157.
Loberg, R. D., Logothetis, C. J., Keller, E. T., & Pienta, K. J. (2005). Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol, 23, 8232–8241.
Guise, T. A., Mohammad, K. S., Clines, G., et al. (2006). Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res, 12, 6213s–6216s.
Kingsley, L. A., Fournier, P. G., Chirgwin, J. M., & Guise, T. A. (2007). Molecular biology of bone metastasis. Mol Cancer Ther, 6, 2609–2617.
Araujo, J., & Logothetis, C. (2009). Targeting Src signaling in metastatic bone disease. Int J Cancer, 124, 1–6.
Soriano, P., Montgomery, C., Geske, R., & Bradley, A. (1991). Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell, 64, 693–702.
Horne, W. C., Sanjay, A., Bruzzaniti, A., & Baron, R. (2005). The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev, 208, 106–125.
Marzia, M., Sims, N. A., Voit, S., et al. (2000). Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol, 151, 311–320.
Lee, Y. C., Huang, C. F., Murshed, M., et al. (2010). Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene, 29, 3196–3207.
Dayyani, F., Varkaris, A., Araujo, J. C., et al. (2013). Increased serum insulin-like growth factor-1 levels are associated with prolonged response to dasatinib-based regimens in metastatic prostate cancer. Prostate, 73, 979–985.
Clezardin, P., & Teti, A. (2007). Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis, 24, 599–608.
Sturge, J., Caley, M. P., & Waxman, J. (2011). Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol, 8, 357–368.
Koreckij, T., Nguyen, H., Brown, L. G., Yu, E. Y., Vessella, R. L., & Corey, E. (2009). Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer, 101, 263–268.
Mukhopadhyay, D., Tsiokas, L., Zhou, X. M., Foster, D., Brugge, J. S., & Sukhatme, V. P. (1995). Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature, 375, 577–581.
Trevino, J. G., Gray, M. J., Nawrocki, S. T., et al. (2006). Src activation of Stat3 is an independent requirement from NF-kappaB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis, 9, 101–110.
Kim, M. P., Park, S. I., Kopetz, S., & Gallick, G. E. (2009). Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis. Cell Tissue Res, 335, 249–259.
Hu, G., Place, A. T., & Minshall, R. D. (2008). Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules. Chem Biol Interact, 171, 177–189.
Sverdlov, M., Shajahan, A. N., & Minshall, R. D. (2007). Tyrosine phosphorylation-dependence of caveolae-mediated endocytosis. J Cell Mol Med, 11, 1239–1250.
Puls, L. N., Eadens, M., & Messersmith, W. (2011). Current status of SRC inhibitors in solid tumor malignancies. Oncologist, 16, 566–578.
Demetri, G. D., Lo, R. P., MacPherson, I. R., et al. (2009). Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res, 15, 6232–6240.
Yu, E. Y., Wilding, G., Posadas, E., et al. (2009). Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res, 15, 7421–7428.
Yu, E. Y., Massard, C., Gross, M. E., et al. (2011). Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology, 77, 1166–1171.
Tannock, I. F., De, W. R., Berry, W. R., et al. (2004). Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med, 351, 1502–1512.
Araujo, J. C., Mathew, P., Armstrong, A. J., et al. (2012). Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer, 118, 63–71.
Araujo, J. C., Trudel, G. C., Saad, F., (2013). et al. Overall survival (OS) and safety of dasatinib/docetaxel versus docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC): results from the randomized phase III READY trial. J Clin Oncol (suppl 6; abstr LBA8)
Baselga, J., Cervantes, A., Martinelli, E., et al. (2010). Phase I safety, pharmacokinetics, and inhibition of SRC activity study of saracatinib in patients with solid tumors. Clin Cancer Res, 16, 4876–4883.
Lara, P. N., Jr., Longmate, J., Evans, C. P., et al. (2009). A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs, 20, 179–184.
Fallah-Tafti, A., Foroumadi, A., Tiwari, R., et al. (2011). Thiazolyl N-benzyl-substituted acetamide derivatives: synthesis, Src kinase inhibitory and anticancer activities. Eur J Med Chem, 46, 4853–4858.
Anbalagan, M., Ali, A., Jones, R. K., et al. (2012). Peptidomimetic Src/pretubulin inhibitor KX-01 alone and in combination with paclitaxel suppresses growth, metastasis in human ER/PR/HER2-negative tumor xenografts. Mol Cancer Ther, 11, 1936–1947.
Naing, A., Cohen, R., Dy, G. K., et al. (2013). A phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket- directed SRC inhibitor, in patients with advanced malignancies. Invest New Drugs, 31, 967–973.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varkaris, A., Katsiampoura, A.D., Araujo, J.C. et al. Src signaling pathways in prostate cancer. Cancer Metastasis Rev 33, 595–606 (2014). https://doi.org/10.1007/s10555-013-9481-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9481-1