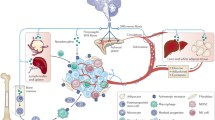
Abstract
Central and sympathetic nervous systems govern functional activities of many organs. Solid tumors like organs are also innervated by sympathetic nerve fibers. Neurotransmitters released from sympathetic nerve fibers can modulate biological behaviors of tumor cells. Multiple physiologic processes of tumor development may be dominated by central and sympathetic nervous systems as well. Recent studies suggest that dysfunction of central and sympathetic nervous systems and disorder of the hormone network induced by psychological stress may influence malignant progression of cancer by inhibiting the functions of immune system, regulating metabolic reprogramming of tumor cells, and inducing interactions between tumor and stromal cells. Over-release of inflammatory cytokines by tumors may aggravate emotional disorder, triggering the vicious cycles in tumor microenvironment and host macroenvironment. It is reasonable to hypothesize that cancer progression may be controlled by central and sympathetic nervous systems. In this review, we will focus on the recent information about the impacts of central and sympathetic nervous systems on tumor invasion and metastasis.




Similar content being viewed by others
References
Hynes, R. O. (2003). Metastatic potential: generic predisposition of the primary tumor or rare, metastatic variants-or both? Cell, 113(7), 821–823.
Nguyen, D. X., & Massague, J. (2007). Genetic determinants of cancer metastasis. Nature Reviews Genetics, 8(5), 341–352.
Ramaswamy, S., Ross, K. N., Lander, E. S., & Golub, T. R. (2003). A molecular signature of metastasis in primary solid tumors. Nature Genetics, 33(1), 49–54.
Hanahan, D., & Coussens, L. M. (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674.
Joyce, J. A., & Pollard, J. W. (2009). Microenvironmental regulation of metastasis. Nature Reviews. Cancer, 9(4), 239–252.
Simon, R. H., Lovett, E. J., 3rd, Tomaszek, D., & Lundy, J. (1980). Electrical stimulation of the midbrain mediates metastatic tumor growth. Science, 209(4461), 1132–1133.
Giraldi, T., Perissin, L., Zorzet, S., Rapozzi, V., & Rodani, M. G. (1994). Metastasis and neuroendocrine system in stressed mice. International Journal of Neuroscience, 74(1–4), 265–278.
Sarkar, D. K., Zhang, C., Murugan, S., Dokur, M., Boyadjieva, N. I., Ortiguela, M., et al. (2011). Transplantation of beta-endorphin neurons into the hypothalamus promotes immune function and restricts the growth and metastasis of mammary carcinoma. Cancer Research, 71(19), 6282–6291.
Sloan, E. K., Priceman, S. J., Cox, B. F., Yu, S., Pimentel, M. A., Tangkanangnukul, V., et al. (2010). The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Research, 70(18), 7042–7052.
Hermes, G. L., Delgado, B., Tretiakova, M., Cavigelli, S. A., Krausz, T., Conzen, S. D., et al. (2009). Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proceedings of the National Academy of Sciences of the United States of America, 106(52), 22393–22398.
Chida, Y., Hamer, M., Wardle, J., & Steptoe, A. (2008). Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature Clinical Practice Oncology, 5(8), 466–475.
Bultz, B. D., & Carlson, L. E. (2005). Emotional distress: the sixth vital sign in cancer care. Journal of Clinical Oncology, 23(26), 6440–6441.
Waller, A., Groff, S. L., Hagen, N., Bultz, B. D., & Carlson, L. E. (2012). Characterizing distress, the 6th vital sign, in an oncology pain clinic. Current Oncology, 19(2), e53–59.
Hsu, P. P., & Sabatini, D. M. (2008). Cancer cell metabolism: Warburg and beyond. Cell, 134(5), 703–707.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033.
Levine, A. J., & Puzio-Kuter, A. M. (2010). The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science, 330(6009), 1340–1344.
Xu, R. H., Pelicano, H., Zhou, Y., Carew, J. S., Feng, L., Bhalla, K. N., et al. (2005). Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Research, 65(2), 613–621.
Pelicano, H., Martin, D. S., Xu, R. H., & Huang, P. (2006). Glycolysis inhibition for anticancer treatment. Oncogene, 25(34), 4633–4646.
Dang, C. V. (2012). Links between metabolism and cancer. Genes & Development, 26(9), 877–890.
Hara, M. R., Kovacs, J. J., Whalen, E. J., Rajagopal, S., Strachan, R. T., Grant, W., et al. (2011). A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature, 477(7364), 349–353.
Hara, M. R., Sachs, B. D., Caron, M. G., & Lefkowitz, R. J. (2013). Pharmacological blockade of a beta(2)AR-beta-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle, 12(2), 219–224.
Vousden, K. H., & Ryan, K. M. (2009). p53 and metabolism. Nature Reviews. Cancer, 9(10), 691–700.
Park, S. Y., Kang, J. H., Jeong, K. J., Lee, J., Han, J. W., Choi, W. S., et al. (2011). Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. International Journal of Cancer, 128(10), 2306–2316.
Denko, N. C. (2008). Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews. Cancer, 8(9), 705–713.
Gerhart-Hines, Z., Dominy, J. E., Jr., Blattler, S. M., Jedrychowski, M. P., Banks, A. S., Lim, J. H., et al. (2011). The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Molecular Cell, 44(6), 851–863.
Brooks, C. L., & Gu, W. (2009). How does SIRT1 affect metabolism, senescence and cancer? Nature Reviews. Cancer, 9(2), 123–128.
Gnaiger, E., Mendez, G., & Hand, S. C. (2000). High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11080–11085.
Heerlein, K., Schulze, A., Hotz, L., Bartsch, P., & Mairbaurl, H. (2005). Hypoxia decreases cellular ATP demand and inhibits mitochondrial respiration of a549 cells. American Journal of Respiratory Cell and Molecular Biology, 32(1), 44–51.
Puka-Sundvall, M., Wallin, C., Gilland, E., Hallin, U., Wang, X., Sandberg, M., et al. (2000). Impairment of mitochondrial respiration after cerebral hypoxia-ischemia in immature rats: relationship to activation of caspase-3 and neuronal injury. Brain Research. Developmental Brain Research, 125(1–2), 43–50.
Keith, B., Johnson, R. S., & Simon, M. C. (2012). HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nature Reviews. Cancer, 12(1), 9–22.
Forsythe, J. A., Jiang, B. H., Iyer, N. V., Agani, F., Leung, S. W., Koos, R. D., et al. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and Cellular Biology, 16(9), 4604–4613.
Raval, R. R., Lau, K. W., Tran, M. G., Sowter, H. M., Mandriota, S. J., Li, J. L., et al. (2005). Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel–Lindau-associated renal cell carcinoma. Molecular and Cellular Biology, 25(13), 5675–5686.
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Nature Reviews. Cancer, 3(10), 721–732.
Guillaumond, F., Leca, J., Olivares, O., Lavaut, M. N., Vidal, N., Berthezene, P., et al. (2013). Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America, 110(10), 3919–3924.
Kim, J. W., Tchernyshyov, I., Semenza, G. L., & Dang, C. V. (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 3(3), 177–185.
Kim, J. W., Gao, P., & Dang, C. V. (2007). Effects of hypoxia on tumor metabolism. Cancer Metastasis Reviews, 26(2), 291–298.
Nonogaki, K. (2000). New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia, 43(5), 533–549.
Rodgers, J. T., Lerin, C., Gerhart-Hines, Z., & Puigserver, P. (2008). Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Letters, 582(1), 46–53.
Wright, P. A., Perry, S. F., & Moon, T. W. (1989). Regulation of hepatic gluconeogenesis and glycogenolysis by catecholamines in rainbow trout during environmental hypoxia. Journal of Experimental Biology, 147, 169–188.
Bartness, T. J., Shrestha, Y. B., Vaughan, C. H., Schwartz, G. J., & Song, C. K. (2010). Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and Cellular Endocrinology, 318(1–2), 34–43.
Hu, H. T., Ma, Q. Y., Zhang, D., Shen, S. G., Han, L., Ma, Y. D., et al. (2010). HIF-1alpha links beta-adrenoceptor agonists and pancreatic cancer cells under normoxic condition. Acta Pharmacologica Sinica, 31(1), 102–110.
Shi, M., Liu, D., Duan, H., Qian, L., Wang, L., Niu, L., et al. (2011). The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Research and Treatment, 125(2), 351–362.
Al-Wadei, M. H., Al-Wadei, H. A., & Schuller, H. M. (2012). Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors alpha3, alpha5, and alpha7. Molecular Cancer Research, 10(2), 239–249.
Canto, C., & Auwerx, J. (2009). Caloric restriction, SIRT1 and longevity. Trends in Endocrinology and Metabolism, 20(7), 325–331.
Michishita, E., Park, J. Y., Burneskis, J. M., Barrett, J. C., & Horikawa, I. (2005). Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Molecular Biology of the Cell, 16(10), 4623–4635.
Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado De Oliveira, R., et al. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature, 429(6993), 771–776.
Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., & Puigserver, P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature, 434(7029), 113–118.
Kersten, S., Desvergne, B., & Wahli, W. (2000). Roles of PPARs in health and disease. Nature, 405(6785), 421–424.
Lefebvre, P., Chinetti, G., Fruchart, J. C., & Staels, B. (2006). Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. The Journal of Clinical Investigation, 116(3), 571–580.
Evans, R. M., Barish, G. D., & Wang, Y. X. (2004). PPARs and the complex journey to obesity. Nature Medicine, 10(4), 355–361.
Lagouge, M., Argmann, C., Gerhart-Hines, Z., Meziane, H., Lerin, C., Daussin, F., et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell, 127(6), 1109–1122.
Lin, J., Handschin, C., & Spiegelman, B. M. (2005). Metabolic control through the PGC-1 family of transcription coactivators. Cell Metabolism, 1(6), 361–370.
Gerhart-Hines, Z., Rodgers, J. T., Bare, O., Lerin, C., Kim, S. H., Mostoslavsky, R., et al. (2007). Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO Journal, 26(7), 1913–1923.
Schwer, B., & Verdin, E. (2008). Conserved metabolic regulatory functions of sirtuins. Cell Metabolism, 7(2), 104–112.
Chalkiadaki, A., & Guarente, L. (2012). Sirtuins mediate mammalian metabolic responses to nutrient availability. Nature Reviews. Endocrinology, 8(5), 287–296.
Nin, V., Escande, C., Chini, C. C., Giri, S., Camacho-Pereira, J., Matalonga, J., et al. (2012). Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase. Journal of Biological Chemistry, 287(28), 23489–23501.
Chao, L. C., & Tontonoz, P. (2012). SIRT1 regulation-it ain’t all NAD. Molecular Cell, 45(1), 9–11.
Canto, C., Gerhart-Hines, Z., Feige, J. N., Lagouge, M., Noriega, L., Milne, J. C., et al. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature, 458(7241), 1056–1060.
Zhang, B. B., Zhou, G., & Li, C. (2009). AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metabolism, 9(5), 407–416.
Antoni, M. H., Lutgendorf, S. K., Cole, S. W., Dhabhar, F. S., Sephton, S. E., McDonald, P. G., et al. (2006). The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews. Cancer, 6(3), 240–248.
Thaker, P. H., Han, L. Y., Kamat, A. A., Arevalo, J. M., Takahashi, R., Lu, C., et al. (2006). Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature Medicine, 12(8), 939–944.
Shi, M., Liu, D., Duan, H., Han, C., Wei, B., Qian, L., et al. (2010). Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Molecular Cancer, 9, 269.
Yang, E. V., Sood, A. K., Chen, M., Li, Y., Eubank, T. D., Marsh, C. B., et al. (2006). Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Research, 66(21), 10357–10364.
Chakroborty, D., Sarkar, C., Basu, B., Dasgupta, P. S., & Basu, S. (2009). Catecholamines regulate tumor angiogenesis. Cancer Research, 69(9), 3727–3730.
Landen, C. N., Jr., Lin, Y. G., Armaiz Pena, G. N., Das, P. D., Arevalo, J. M., Kamat, A. A., et al. (2007). Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Research, 67(21), 10389–10396.
Shahzad, M. M., Arevalo, J. M., Armaiz-Pena, G. N., Lu, C., Stone, R. L., Moreno-Smith, M., et al. (2010). Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. Journal of Biological Chemistry, 285(46), 35462–35470.
Yang, E. V., Kim, S. J., Donovan, E. L., Chen, M., Gross, A. C., Webster Marketon, J. I., et al. (2009). Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain, Behavior, and Immunity, 23(2), 267–275.
Wolf, J. M., Rohleder, N., Bierhaus, A., Nawroth, P. P., & Kirschbaum, C. (2009). Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain, Behavior, and Immunity, 23(6), 742–749.
Qian, B. Z., & Pollard, J. W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141(1), 39–51.
Mantovani, A., Schioppa, T., Porta, C., Allavena, P., & Sica, A. (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Reviews, 25(3), 315–322.
Entschladen, F., Drell, T. L. T., Lang, K., Joseph, J., & Zaenker, K. S. (2004). Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. The Lancet Oncology, 5(4), 254–258.
Ruff, M., Schiffmann, E., Terranova, V., & Pert, C. B. (1985). Neuropeptides are chemoattractants for human tumor cells and monocytes: a possible mechanism for metastasis. Clinical Immunology and Immunopathology, 37(3), 387–396.
Barron, T. I., Sharp, L., & Visvanathan, K. (2012). Beta-adrenergic blocking drugs in breast cancer: a perspective review. Ther Adv Med Oncol, 4(3), 113–125.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–867.
Lutgendorf, S. K., Lamkin, D. M., Jennings, N. B., Arevalo, J. M., Penedo, F., DeGeest, K., et al. (2008). Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clinical Cancer Research, 14(21), 6839–6846.
Moreno-Smith, M., Lutgendorf, S. K., & Sood, A. K. (2010). Impact of stress on cancer metastasis. Future Oncology, 6(12), 1863–1881.
Vazquez, S. M., Pignataro, O., & Luthy, I. A. (1999). Alpha2-adrenergic effect on human breast cancer MCF-7 cells. Breast Cancer Research and Treatment, 55(1), 41–49.
Huang, H. H., Brennan, T. C., Muir, M. M., & Mason, R. S. (2009). Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. Journal of Cellular Physiology, 220(1), 267–275.
Bruzzone, A., Pinero, C. P., Rojas, P., Romanato, M., Gass, H., Lanari, C., et al. (2011). alpha(2)-Adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Current Cancer Drug Targets, 11(6), 763–774.
Cao, L., Liu, X., Lin, E. J., Wang, C., Choi, E. Y., Riban, V., et al. (2010). Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell, 142(1), 52–64.
Irwin, M. R., & Cole, S. W. (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625–632.
Glaser, R., & Kiecolt-Glaser, J. K. (2005). Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology, 5(3), 243–251.
Padgett, D. A., & Glaser, R. (2003). How stress influences the immune response. Trends in Immunology, 24(8), 444–448.
Stewart, J., Meaney, M. J., Aitken, D., Jensen, L., & Kalant, N. (1988). The effects of acute and life-long food restriction on basal and stress-induced serum corticosterone levels in young and aged rats. Endocrinology, 123(4), 1934–1941.
Padgett, D. A., Marucha, P. T., & Sheridan, J. F. (1998). Restraint stress slows cutaneous wound healing in mice. Brain, Behavior, and Immunity, 12(1), 64–73.
Elenkov, I. J. (2004). Glucocorticoids and the Th1/Th2 balance. Annals of the New York Academy of Sciences, 1024, 138–146.
Liu, B., Li, Z., Mahesh, S. P., Pantanelli, S., Hwang, F. S., Siu, W. O., et al. (2008). Glucocorticoid-induced tumor necrosis factor receptor negatively regulates activation of human primary natural killer (NK) cells by blocking proliferative signals and increasing NK cell apoptosis. Journal of Biological Chemistry, 283(13), 8202–8210.
Sephton, S. E., Sapolsky, R. M., Kraemer, H. C., & Spiegel, D. (2000). Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute, 92(12), 994–1000.
Abercrombie, H. C., Giese-Davis, J., Sephton, S., Epel, E. S., Turner-Cobb, J. M., & Spiegel, D. (2004). Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology, 29(8), 1082–1092.
Irwin, M., Daniels, M., Risch, S. C., Bloom, E., & Weiner, H. (1988). Plasma cortisol and natural killer cell activity during bereavement. Biological Psychiatry, 24(2), 173–178.
Irwin, M., Daniels, M., Smith, T. L., Bloom, E., & Weiner, H. (1987). Impaired natural killer cell activity during bereavement. Brain, Behavior, and Immunity, 1(1), 98–104.
Elenkov, I. J., Chrousos, G. P., & Wilder, R. L. (2000). Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Annals of the New York Academy of Sciences, 917, 94–105.
Franchimont, D., Galon, J., Gadina, M., Visconti, R., Zhou, Y., Aringer, M., et al. (2000). Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. Journal of Immunology, 164(4), 1768–1774.
Blotta, M. H., DeKruyff, R. H., & Umetsu, D. T. (1997). Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. Journal of Immunology, 158(12), 5589–5595.
Auphan, N., DiDonato, J. A., Rosette, C., Helmberg, A., & Karin, M. (1995). Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science, 270(5234), 286–290.
Scheinman, R. I., Cogswell, P. C., Lofquist, A. K., & Baldwin, A. S., Jr. (1995). Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science, 270(5234), 283–286.
Ray, A., & Prefontaine, K. E. (1994). Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America, 91(2), 752–756.
Franchimont, D. (2004). Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Annals of the New York Academy of Sciences, 1024, 124–137.
Swanson, M. A., Lee, W. T., & Sanders, V. M. (2001). IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. Journal of Immunology, 166(1), 232–240.
Elenkov, I. J., Papanicolaou, D. A., Wilder, R. L., & Chrousos, G. P. (1996). Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proceedings of the Association of American Physicians, 108(5), 374–381.
Stevenson, J. R., Westermann, J., Liebmann, P. M., Hortner, M., Rinner, I., Felsner, P., et al. (2001). Prolonged alpha-adrenergic stimulation causes changes in leukocyte distribution and lymphocyte apoptosis in the rat. Journal of Neuroimmunology, 120(1–2), 50–57.
Jiang, J. L., Peng, Y. P., Qiu, Y. H., & Wang, J. J. (2007). Effect of endogenous catecholamines on apoptosis of Con A-activated lymphocytes of rats. Journal of Neuroimmunology, 192(1–2), 79–88.
Elenkov, I. J., & Chrousos, G. P. (2002). Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Annals of the New York Academy of Sciences, 966, 290–303.
Schedlowski, M., Falk, A., Rohne, A., Wagner, T. O., Jacobs, R., Tewes, U., et al. (1993). Catecholamines induce alterations of distribution and activity of human natural killer (NK) cells. Journal of Clinical Immunology, 13(5), 344–351.
Crary, B., Hauser, S. L., Borysenko, M., Kutz, I., Hoban, C., Ault, K. A., et al. (1983). Epinephrine-induced changes in the distribution of lymphocyte subsets in peripheral blood of humans. Journal of Immunology, 131(3), 1178–1181.
Kalinichenko, V. V., Mokyr, M. B., Graf, L. H., Jr., Cohen, R. L., & Chambers, D. A. (1999). Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. Journal of Immunology, 163(5), 2492–2499.
Straub, R. H., Mayer, M., Kreutz, M., Leeb, S., Scholmerich, J., & Falk, W. (2000). Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. Journal of Leukocyte Biology, 67(4), 553–558.
Neves, S. R., Ram, P. T., & Iyengar, R. (2002). G protein pathways. Science, 296(5573), 1636–1639.
Audet, M., & Bouvier, M. (2012). Restructuring G-protein-coupled receptor activation. Cell, 151(1), 14–23.
Lutgendorf, S. K., Sood, A. K., & Antoni, M. H. (2010). Host factors and cancer progression: biobehavioral signaling pathways and interventions. Journal of Clinical Oncology, 28(26), 4094–4099.
Cole, S. W., & Sood, A. K. (2012). Molecular pathways: beta-adrenergic signaling in cancer. Clinical Cancer Research, 18(5), 1201–1206.
Hassan, S., Karpova, Y., Baiz, D., Yancey, D., Pullikuth, A., Flores, A., et al. (2013). Behavioral stress accelerates prostate cancer development in mice. J Clin Invest, 123(2), 874–886.
Armaiz-Pena, G. N., Allen, J. K., Cruz, A., Stone, R. L., Nick, A. M., Lin, Y. G., et al. (2013). Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nature Communications, 4, 1403.
Sethi, N., & Kang, Y. (2011). Unravelling the complexity of metastasis—molecular understanding and targeted therapies. Nature Reviews. Cancer, 11(10), 735–748.
Rangarajan, S., Enserink, J. M., Kuiperij, H. B., de Rooij, J., Price, L. S., Schwede, F., et al. (2003). Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. The Journal of Cell Biology, 160(4), 487–493.
Fukuhara, S., Sakurai, A., Sano, H., Yamagishi, A., Somekawa, S., Takakura, N., et al. (2005). Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Molecular and Cellular Biology, 25(1), 136–146.
Enserink, J. M., Price, L. S., Methi, T., Mahic, M., Sonnenberg, A., Bos, J. L., et al. (2004). The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. Journal of Biological Chemistry, 279(43), 44889–44896.
Ma, X., Zhao, Y., Daaka, Y., & Nie, Z. (2012). Acute activation of beta2-adrenergic receptor regulates focal adhesions through betaArrestin2- and p115RhoGEF protein-mediated activation of RhoA. Journal of Biological Chemistry, 287(23), 18925–18936.
Pham, H., Chen, M., Takahashi, H., King, J., Reber, H. A., Hines, O. J., et al. (2012). Apigenin inhibits NNK-induced focal adhesion kinase activation in pancreatic cancer cells. Pancreas, 41(8), 1306–1315.
Masur, K., Niggemann, B., Zanker, K. S., & Entschladen, F. (2001). Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Research, 61(7), 2866–2869.
Lang, K., Drell, T. L. T., Lindecke, A., Niggemann, B., Kaltschmidt, C., Zaenker, K. S., et al. (2004). Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. International Journal of Cancer, 112(2), 231–238.
Drell, T. L. T., Joseph, J., Lang, K., Niggemann, B., Zaenker, K. S., & Entschladen, F. (2003). Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Research and Treatment, 80(1), 63–70.
Boulay, G., Malaquin, N., Loison, I., Foveau, B., Van Rechem, C., Rood, B. R., et al. (2012). Loss of hypermethylated in cancer 1 (HIC1) in breast cancer cells contributes to stress-induced migration and invasion through beta-2 adrenergic receptor (ADRB2) misregulation. Journal of Biological Chemistry, 287(8), 5379–5389.
Sood, A. K., Bhatty, R., Kamat, A. A., Landen, C. N., Han, L., Thaker, P. H., et al. (2006). Stress hormone-mediated invasion of ovarian cancer cells. Clinical Cancer Research, 12(2), 369–375.
Palm, D., Lang, K., Niggemann, B., Drell, T. L. T., Masur, K., Zaenker, K. S., et al. (2006). The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. International Journal of Cancer, 118(11), 2744–2749.
Taddei, M. L., Giannoni, E., Fiaschi, T., & Chiarugi, P. (2012). Anoikis: an emerging hallmark in health and diseases. The Journal of Pathology, 226(2), 380–393.
Keledjian, K., & Kyprianou, N. (2003). Anoikis induction by quinazoline based alpha 1-adrenoceptor antagonists in prostate cancer cells: antagonistic effect of bcl-2. Journal of Urology, 169(3), 1150–1156.
Johansson, M. (2011). Lord of the rings: a promising novel treatment for renal cell carcinoma? European Urology, 59(5), 745–746.
Sakamoto, S., Schwarze, S., & Kyprianou, N. (2011). Anoikis disruption of focal adhesion-Akt signaling impairs renal cell carcinoma. European Urology, 59(5), 734–744.
Alberti, C. (2007). Apoptosis induction by quinazoline-derived alpha1-blockers in prostate cancer cells: biomolecular implications and clinical relevance. European Review for Medical and Pharmacological Sciences, 11(1), 59–64.
Benning, C. M., & Kyprianou, N. (2002). Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. Cancer Research, 62(2), 597–602.
Sood, A. K., Armaiz-Pena, G. N., Halder, J., Nick, A. M., Stone, R. L., Hu, W., et al. (2010). Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. The Journal of Clinical Investigation, 120(5), 1515–1523.
Sastry, K. S., Karpova, Y., Prokopovich, S., Smith, A. J., Essau, B., Gersappe, A., et al. (2007). Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. Journal of Biological Chemistry, 282(19), 14094–14100.
Strell, C., Niggemann, B., Voss, M. J., Powe, D. G., Zanker, K. S., & Entschladen, F. (2012). Norepinephrine promotes the beta1-integrin-mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROalpha release. Molecular Cancer Research, 10(2), 197–207.
Bernot, D., Peiretti, F., Canault, M., Juhan-Vague, I., & Nalbone, G. (2005). Upregulation of TNF-alpha-induced ICAM-1 surface expression by adenylate cyclase-dependent pathway in human endothelial cells. Journal of Cellular Physiology, 202(2), 434–441.
Pozgajova, M., Sachs, U. J., Hein, L., & Nieswandt, B. (2006). Reduced thrombus stability in mice lacking the alpha2A-adrenergic receptor. Blood, 108(2), 510–514.
Abecassis, J., Millon-Collard, R., Klein-Soyer, C., Nicora, F., Fricker, J. P., Beretz, A., et al. (1987). Adhesion of human breast cancer cell line MCF-7 to human vascular endothelial cells in culture. Enhancement by activated platelets. International Journal of Cancer, 40(4), 525–531.
Kaplan, R. N., Rafii, S., & Lyden, D. (2006). Preparing the "soil": the premetastatic niche. Cancer Research, 66(23), 11089–11093.
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Reviews. Cancer, 3(6), 453–458.
Sloan, E. K., Capitanio, J. P., Tarara, R. P., Mendoza, S. P., Mason, W. A., & Cole, S. W. (2007). Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. Journal of Neuroscience, 27(33), 8857–8865.
Tang, Y., Shankar, R., Gamelli, R., & Jones, S. (1999). Dynamic norepinephrine alterations in bone marrow: evidence of functional innervation. Journal of Neuroimmunology, 96(2), 182–189.
Elefteriou, F. (2005). Neuronal signaling and the regulation of bone remodeling. Cellular and Molecular Life Sciences, 62(19–20), 2339–2349.
Takeda, S., Elefteriou, F., Levasseur, R., Liu, X., Zhao, L., Parker, K. L., et al. (2002). Leptin regulates bone formation via the sympathetic nervous system. Cell, 111(3), 305–317.
Campbell, J. P., Karolak, M. R., Ma, Y., Perrien, D. S., Masood-Campbell, S. K., Penner, N. L., et al. (2012). Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biology, 10(7), e1001363.
Epstein, R. J. (2004). The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nature Reviews. Cancer, 4(11), 901–909.
Roussos, E. T., Condeelis, J. S., & Patsialou, A. (2011). Chemotaxis in cancer. Nature Reviews. Cancer, 11(8), 573–587.
Subik, K., Shu, L., Wu, C., Liang, Q., Hicks, D., Boyce, B., et al. (2012). The ubiquitin E3 ligase WWP1 decreases CXCL12-mediated MDA231 breast cancer cell migration and bone metastasis. Bone, 50(4), 813–823.
Katayama, Y., Battista, M., Kao, W. M., Hidalgo, A., Peired, A. J., Thomas, S. A., et al. (2006). Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell, 124(2), 407–421.
Dar, A., Schajnovitz, A., Lapid, K., Kalinkovich, A., Itkin, T., Ludin, A., et al. (2011). Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia, 25(8), 1286–1296.
Mendez-Ferrer, S., Battista, M., & Frenette, P. S. (2010). Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Annals of the New York Academy of Sciences, 1192, 139–144.
Rozniecki, J. J., Sahagian, G. G., Kempuraj, D., Tao, K., Jocobson, S., Zhang, B., et al. (2010). Brain metastases of mouse mammary adenocarcinoma is increased by acute stress. Brain Research, 1366, 204–210.
Theoharides, T. C., Rozniecki, J. J., Sahagian, G., Jocobson, S., Kempuraj, D., Conti, P., et al. (2008). Impact of stress and mast cells on brain metastases. Journal of Neuroimmunology, 205(1–2), 1–7.
Li, N., Grivennikov, S. I., & Karin, M. (2011). The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell, 19(4), 429–431.
Deng, J., Liu, Y., Lee, H., Herrmann, A., Zhang, W., Zhang, C., et al. (2012). S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell, 21(5), 642–654.
Lutgendorf, S. K., De Geest, K., Bender, D., Ahmed, A., Goodheart, M. J., Dahmoush, L., et al. (2012). Social influences on clinical outcomes of patients with ovarian cancer. Journal of Clinical Oncology, 30(23), 2885–2890.
Andersen, B. L., Yang, H. C., Farrar, W. B., Golden-Kreutz, D. M., Emery, C. F., Thornton, L. M., et al. (2008). Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer, 113(12), 3450–3458.
Lengacher, C. A., Johnson-Mallard, V., Post-White, J., Moscoso, M. S., Jacobsen, P. B., Klein, T. W., et al. (2009). Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-Oncology, 18(12), 1261–1272.
Hoffman, C. J., Ersser, S. J., Hopkinson, J. B., Nicholls, P. G., Harrington, J. E., & Thomas, P. W. (2012). Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. Journal of Clinical Oncology, 30(12), 1335–1342.
Coyne, J. C., & Tennen, H. (2010). Positive psychology in cancer care: bad science, exaggerated claims, and unproven medicine. Annals of Behavioral Medicine, 39(1), 16–26.
Stefanek, M. E., Palmer, S. C., Thombs, B. D., & Coyne, J. C. (2009). Finding what is not there: unwarranted claims of an effect of psychosocial intervention on recurrence and survival. Cancer, 115(24), 5612–5616.
Dantzer, R., Konsman, J. P., Bluthe, R. M., & Kelley, K. W. (2000). Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Autonomic Neuroscience, 85(1–3), 60–65.
de Vries, H. E., Blom-Roosemalen, M. C., van Oosten, M., de Boer, A. G., van Berkel, T. J., Breimer, D. D., et al. (1996). The influence of cytokines on the integrity of the blood–brain barrier in vitro. Journal of Neuroimmunology, 64(1), 37–43.
Huber, J. D., Egleton, R. D., & Davis, T. P. (2001). Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends in Neurosciences, 24(12), 719–725.
Theoharides, T. C., & Konstantinidou, A. D. (2007). Corticotropin-releasing hormone and the blood–brain-barrier. Frontiers in Bioscience, 12, 1615–1628.
Banks, W. A., Kastin, A. J., & Broadwell, R. D. (1995). Passage of cytokines across the blood–brain barrier. Neuroimmunomodulation, 2(4), 241–248.
Ek, M., Kurosawa, M., Lundeberg, T., & Ericsson, A. (1998). Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. Journal of Neuroscience, 18(22), 9471–9479.
Dantzer, R. (2006). Cytokine, sickness behavior, and depression. Neurologic Clinics, 24(3), 441–460.
Sukoff Rizzo, S. J., Neal, S. J., Hughes, Z. A., Beyna, M., Rosenzweig-Lipson, S., Moss, S. J., et al. (2012). Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry, 2, e199.
Kaster, M. P., Gadotti, V. M., Calixto, J. B., Santos, A. R., & Rodrigues, A. L. (2012). Depressive-like behavior induced by tumor necrosis factor-alpha in mice. Neuropharmacology, 62(1), 419–426.
Pechnick, R. N., Chesnokova, V. M., Kariagina, A., Price, S., Bresee, C. J., & Poland, R. E. (2004). Reduced immobility in the forced swim test in mice with a targeted deletion of the leukemia inhibitory factor (LIF) gene. Neuropsychopharmacology, 29(4), 770–776.
Lamkin, D. M., Lutgendorf, S. K., Lubaroff, D., Sood, A. K., Beltz, T. G., & Johnson, A. K. (2011). Cancer induces inflammation and depressive-like behavior in the mouse: modulation by social housing. Brain, Behavior, and Immunity, 25(3), 555–564.
Pyter, L. M., Pineros, V., Galang, J. A., McClintock, M. K., & Prendergast, B. J. (2009). Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proceedings of the National Academy of Sciences of the United States of America, 106(22), 9069–9074.
Musselman, D. L., Miller, A. H., Porter, M. R., Manatunga, A., Gao, F., Penna, S., et al. (2001). Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. The American Journal of Psychiatry, 158(8), 1252–1257.
Lutgendorf, S. K., Weinrib, A. Z., Penedo, F., Russell, D., DeGeest, K., Costanzo, E. S., et al. (2008). Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. Journal of Clinical Oncology, 26(29), 4820–4827.
Costanzo, E. S., Lutgendorf, S. K., Sood, A. K., Anderson, B., Sorosky, J., & Lubaroff, D. M. (2005). Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer, 104(2), 305–313.
Gola, H., Engler, H., Sommershof, A., Adenauer, H., Kolassa, S., Schedlowski, M., et al. (2013). Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry, 13, 40.
Sutherland, A. G., Alexander, D. A., & Hutchison, J. D. (2003). Disturbance of pro-inflammatory cytokines in post-traumatic psychopathology. Cytokine, 24(5), 219–225.
Garcia-Oscos, F., Salgado, H., Hall, S., Thomas, F., Farmer, G. E., Bermeo, J., et al. (2012). The stress-induced cytokine interleukin-6 decreases the inhibition/excitation ratio in the rat temporal cortex via trans-signaling. Biological Psychiatry, 71(7), 574–582.
Nilsson, M. B., Armaiz-Pena, G., Takahashi, R., Lin, Y. G., Trevino, J., Li, Y., et al. (2007). Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. Journal of Biological Chemistry, 282(41), 29919–29926.
Lutgendorf, S. K., DeGeest, K., Dahmoush, L., Farley, D., Penedo, F., Bender, D., et al. (2011). Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain, Behavior, and Immunity, 25(2), 250–255.
Lutgendorf, S. K., DeGeest, K., Sung, C. Y., Arevalo, J. M., Penedo, F., Lucci, J., 3rd, et al. (2009). Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain, Behavior, and Immunity, 23(2), 176–183.
Nguyen, K. D., Qiu, Y., Cui, X., Goh, Y. P., Mwangi, J., David, T., et al. (2011). Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature, 480(7375), 104–108.
Flierl, M. A., Rittirsch, D., Nadeau, B. A., Chen, A. J., Sarma, J. V., Zetoune, F. S., et al. (2007). Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature, 449(7163), 721–725.
Weil-Malherbe, H., & Bone, A. D. (1954). Blood platelets as carriers of adrenaline and noradrenaline. Nature, 174(4429), 557–558.
Basu, S., Nagy, J. A., Pal, S., Vasile, E., Eckelhoefer, I. A., Bliss, V. S., et al. (2001). The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nature Medicine, 7(5), 569–574.
Chakroborty, D., Sarkar, C., Mitra, R. B., Banerjee, S., Dasgupta, P. S., & Basu, S. (2004). Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clinical Cancer Research, 10(13), 4349–4356.
Moreno-Smith, M., Lu, C., Shahzad, M. M., Pena, G. N., Allen, J. K., Stone, R. L., et al. (2011). Dopamine blocks stress-mediated ovarian carcinoma growth. Clinical Cancer Research, 17(11), 3649–3659.
Basu, S., Sarkar, C., Chakroborty, D., Nagy, J., Mitra, R. B., Dasgupta, P. S., et al. (2004). Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Research, 64(16), 5551–5555.
Sarkar, C., Chakroborty, D., Chowdhury, U. R., Dasgupta, P. S., & Basu, S. (2008). Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clinical Cancer Research, 14(8), 2502–2510.
Chakroborty, D., Sarkar, C., Yu, H., Wang, J., Liu, Z., Dasgupta, P. S., et al. (2011). Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 108(51), 20730–20735.
Seeman, P., & Lee, T. (1975). Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science, 188(4194), 1217–1219.
Amson, R., Pece, S., Lespagnol, A., Vyas, R., Mazzarol, G., Tosoni, D., et al. (2012). Reciprocal repression between P53 and TCTP. Nature Medicine, 18(1), 91–99.
Kang, S., Dong, S. M., Kim, B. R., Park, M. S., Trink, B., Byun, H. J., et al. (2012). Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis, 17(9), 989–997.
Sachlos, E., Risueno, R. M., Laronde, S., Shapovalova, Z., Lee, J. H., Russell, J., et al. (2012). Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell, 149(6), 1284–1297.
Liu, K., & Ding, S. (2012). Target practice: modeling tumors with stem cells. Cell, 149(6), 1185–1187.
Burgess, D. J. (2012). Stem cells. Antipsychotic to anticancer agent. Nature Reviews. Cancer, 12(7), 452–453.
Burgess, D. J. (2012). Anticancer drugs: antipsychotic to anticancer agent? Nature Reviews. Drug Discovery, 11(7), 516.
Bilkei-Gorzo, A., & Zimmer, A. (2005). Mutagenesis and knockout models: NK1 and substance P. Handbook of Experimental Pharmacology, 169, 143–162.
Esteban, F., Munoz, M., Gonzalez-Moles, M. A., & Rosso, M. (2006). A role for substance P in cancer promotion and progression: a mechanism to counteract intracellular death signals following oncogene activation or DNA damage. Cancer Metastasis Reviews, 25(1), 137–145.
Rosso, M., Munoz, M., & Berger, M. (2012). The role of neurokinin-1 receptor in the microenvironment of inflammation and cancer. ScientificWorldJournal, 2012, 381434.
Rao, G., Patel, P. S., Idler, S. P., Maloof, P., Gascon, P., Potian, J. A., et al. (2004). Facilitating role of preprotachykinin-I gene in the integration of breast cancer cells within the stromal compartment of the bone marrow: a model of early cancer progression. Cancer Research, 64(8), 2874–2881.
Singh, D., Joshi, D. D., Hameed, M., Qian, J., Gascon, P., Maloof, P. B., et al. (2000). Increased expression of preprotachykinin-I and neurokinin receptors in human breast cancer cells: implications for bone marrow metastasis. Proceedings of the National Academy of Sciences of the United States of America, 97(1), 388–393.
Castro, T. A., Cohen, M. C., & Rameshwar, P. (2005). The expression of neurokinin-1 and preprotachykinin-1 in breast cancer cells depends on the relative degree of invasive and metastatic potential. Clinical & Experimental Metastasis, 22(8), 621–628.
Li, X., Ma, G., Ma, Q., Li, W., Liu, J., Han, L., et al. (2013). Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Molecular Cancer Research, 11(3), 294–302.
Munoz, M., Martinez-Armesto, J., & Covenas, R. (2012). NK-1 receptor antagonists as antitumor drugs: a survey of the literature from 2000 to 2011. Expert Opinion on Therapeutic Patents, 22(7), 735–746.
Munoz, M., & Covenas, R. (2012). NK-1 receptor antagonists: a new generation of anticancer drugs. Mini Reviews in Medicinal Chemistry, 12(7), 593–599.
Munoz, M., Rosso, M., & Covenas, R. (2011). The NK-1 receptor: a new target in cancer therapy. Current Drug Targets, 12(6), 909–921.
Pedrazzini, T., Pralong, F., & Grouzmann, E. (2003). Neuropeptide Y: the universal soldier. Cellular and Molecular Life Sciences, 60(2), 350–377.
Kuo, L. E., Kitlinska, J. B., Tilan, J. U., Li, L., Baker, S. B., Johnson, M. D., et al. (2007). Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nature Medicine, 13(7), 803–811.
Kitlinska, J. (2007). Neuropeptide Y in neural crest-derived tumors: effect on growth and vascularization. Cancer Letters, 245(1–2), 293–302.
Gilaberte, Y., Roca, M. J., Garcia-Prats, M. D., Coscojuela, C., Arbues, M. D., & Vera-Alvarez, J. J. (2012). Neuropeptide Y expression in cutaneous melanoma. Journal of the American Academy of Dermatology, 66(6), e201–208.
Korner, M., & Reubi, J. C. (2007). NPY receptors in human cancer: a review of current knowledge. Peptides, 28(2), 419–425.
Kitlinska, J., Abe, K., Kuo, L., Pons, J., Yu, M., Li, L., et al. (2005). Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Research, 65(5), 1719–1728.
Reubi, J. C., Gugger, M., Waser, B., & Schaer, J. C. (2001). Y(1)-mediated effect of neuropeptide Y in cancer: breast carcinomas as targets. Cancer Research, 61(11), 4636–4641.
Korner, M., Waser, B., & Reubi, J. C. (2004). Neuropeptide Y receptor expression in human primary ovarian neoplasms. Laboratory Investigation, 84(1), 71–80.
Sheriff, S., Ali, M., Yahya, A., Haider, K. H., Balasubramaniam, A., & Amlal, H. (2010). Neuropeptide Y Y5 receptor promotes cell growth through extracellular signal-regulated kinase signaling and cyclic AMP inhibition in a human breast cancer cell line. Molecular Cancer Research, 8(4), 604–614.
Medeiros, P. J., Al-Khazraji, B. K., Novielli, N. M., Postovit, L. M., Chambers, A. F., & Jackson, D. N. (2012). Neuropeptide Y stimulates proliferation and migration in the 4T1 breast cancer cell line. International Journal of Cancer, 131(2), 276–286.
Singer, K., Morris, D. L., Oatmen, K. E., Wang, T., Delproposto, J., Mergian, T., et al. (2013). Neuropeptide y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS One, 8(3), e57929.
Kuo, L. E., Czarnecka, M., Kitlinska, J. B., Tilan, J. U., Kvetnansky, R., & Zukowska, Z. (2008). Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Annals of the New York Academy of Sciences, 1148, 232–237.
Han, R., Kitlinska, J. B., Munday, W. R., Gallicano, G. I., & Zukowska, Z. (2012). Stress hormone epinephrine enhances adipogenesis in murine embryonic stem cells by up-regulating the neuropeptide Y system. PLoS One, 7(5), e36609.
Park, E. J., Lee, J. H., Yu, G. Y., He, G., Ali, S. R., Holzer, R. G., et al. (2010). Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell, 140(2), 197–208.
Khandekar, M. J., Cohen, P., & Spiegelman, B. M. (2011). Molecular mechanisms of cancer development in obesity. Nature Reviews. Cancer, 11(12), 886–895.
Calle, E. E., & Kaaks, R. (2004). Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews. Cancer, 4(8), 579–591.
Saavedra, J. M., & Benicky, J. (2007). Brain and peripheral angiotensin II play a major role in stress. Stress, 10(2), 185–193.
Yang, G., Wan, Y., & Zhu, Y. (1996). Angiotensin II—an important stress hormone. Biological Signals, 5(1), 1–8.
Yang, G., Xi, Z. X., Wan, Y., Wang, H., & Bi, G. (1993). Changes in circulating and tissue angiotensin II during acute and chronic stress. Biological Signals, 2(3), 166–172.
George, A. J., Thomas, W. G., & Hannan, R. D. (2010). The renin-angiotensin system and cancer: old dog, new tricks. Nature Reviews. Cancer, 10(11), 745–759.
Rodrigues-Ferreira, S., Abdelkarim, M., Dillenburg-Pilla, P., Luissint, A. C., di-Tommaso, A., Deshayes, F., et al. (2012). Angiotensin II facilitates breast cancer cell migration and metastasis. PLoS One, 7(4), e35667.
Chen, X., Meng, Q., Zhao, Y., Liu, M., Li, D., Yang, Y., et al. (2013). Angiotensin II type 1 receptor antagonists inhibit cell proliferation and angiogenesis in breast cancer. Cancer Letters, 328(2), 318–324.
Napoleone, E., Cutrone, A., Cugino, D., Amore, C., Di Santo, A., Iacoviello, L., et al. (2012). Inhibition of the renin–angiotensin system downregulates tissue factor and vascular endothelial growth factor in human breast carcinoma cells. Thrombosis Research, 129(6), 736–742.
Keizman, D., Huang, P., Eisenberger, M. A., Pili, R., Kim, J. J., Antonarakis, E. S., et al. (2011). Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. European Journal of Cancer, 47(13), 1955–1961.
Amano, H., Ito, Y., Ogawa, F., Eshima, K., Suzuki, T., Oba, K., et al. (2013). Angiotensin II type 1A receptor signaling facilitates tumor metastasis formation through P-selectin-mediated interaction of tumor cells with platelets and endothelial cells. American Journal of Pathology, 182(2), 553–564.
Okamoto, K., Tajima, H., Nakanuma, S., Sakai, S., Makino, I., Kinoshita, J., et al. (2012). Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. International Journal of Oncology, 41(2), 573–582.
Okamoto, K., Tajima, H., Ohta, T., Nakanuma, S., Hayashi, H., Nakagawara, H., et al. (2010). Angiotensin II induces tumor progression and fibrosis in intrahepatic cholangiocarcinoma through an interaction with hepatic stellate cells. International Journal of Oncology, 37(5), 1251–1259.
Cortez-Retamozo, V., Etzrodt, M., Newton, A., Ryan, R., Pucci, F., Sio, S. W., et al. (2013). Angiotensin II drives the production of tumor-promoting macrophages. Immunity, 38(2), 296–308.
Gabrilovich, D. I., Ostrand-Rosenberg, S., & Bronte, V. (2012). Coordinated regulation of myeloid cells by tumours. Nature Reviews Immunology, 12(4), 253–268.
Gabrilovich, D. I. (2013). Applying pressure on macrophages. Immunity, 38(2), 205–206.
Acknowledgments
This work is supported by the National Basic Research Program of China (973 program, no. 2010CB911904), National Natural Science Foundation of China (no. 30901766 and 30972690 and 30800582), and Beijing Natural Science Foundation (no. 7122124 and 7132163).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shi, M., Liu, D., Yang, Z. et al. Central and peripheral nervous systems: master controllers in cancer metastasis. Cancer Metastasis Rev 32, 603–621 (2013). https://doi.org/10.1007/s10555-013-9440-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9440-x