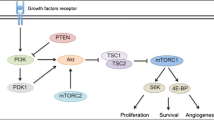
Abstract
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are rare neoplasms that require a multidisciplinary approach for an optimal management. The lack of effectiveness of traditional DNA-damaging agents has led to the exploration of new targeted drugs in order to exploit phenotypical features of GEP-NET therapy. However, due to the orphan setting of these tumors, deeper characterization of molecular features and pathways that characterize cell growth, apoptosis, angiogenesis, and invasion are lacking, particularly genetic mutations or epigenetic alterations that generate oncogenic dependency or even addiction. The PI3K-AKT-mTOR pathway has been implicated as having a crucial role in GEP-NETs not only due to the overexpression of several growth factors and their receptors that finally activate this axis but also hereditary syndromes with constitutive activation of the mTOR pathway with high incidence of GEP-NETs. In this article, we aim to review the recent development of the main molecules that target mTOR complex and have showed promising activity in the treatment of GEPNETs.
Similar content being viewed by others
References
Hansel, D. E., Rahman, A., Hermans, J., et al. (2003). Liver metastases arising from well-differentiated pancreatic endocrine neoplasms demonstrate increased VEGF-C expression. Modern Pathology, 16(7), 652–659.
Zhang, J., Jia, Z., Li, Q., et al. (2007). Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer, 109(8), 1478–1486.
Furukawa, M., Raffeld, M., Mateo, C., et al. (2005). Increased expression of insulin-like growth factor I and/or its receptor in gastrinomas is associated with low curability, increased growth, and development of metastases. Clinical Cancer Research, 11(9), 3233–3242.
Chaudhry, A., Papanicolaou, V., Oberg, K., et al. (1992). Expression of platelet-derived growth factor and its receptors in neuroendocrine tumors of the digestive system. Cancer Research, 52(4), 1006–1012.
Nilsson, O., Wangberg, B., Kolby, L., et al. (1995). Expression of transforming growth factor alpha and its receptor in human neuroendocrine tumours. International Journal of Cancer, 60(5), 645–651.
Fjällskog, M. L., Lejonklou, M. H., Oberg, K. E., et al. (2003). Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clinical Cancer Research, 9(4), 1469–1473.
Wulbrand, U., Remmert, G., Zofel, P., et al. (2000). mRNA expression patterns of insulin-like growth factor system components in human neuroendocrine tumours. European Journal of Clinical Investigation, 30(8), 729–739.
Kaltsas, G. A., Besser, G. M., & Grossman, A. B. (2004). The diagnosis and medical management of advanced neuroendocrine tumors. Endocrine Reviews, 25(3), 458–511.
O’Toole, D., Couvelard, A., Rebours, V., et al. (2010). Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocrine-Related Cancer, 17(4), 847–856.
Kolby, L., Persson, G., Franzen, S., et al. (2003). Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. The British Journal of Surgery, 90(6), 687–693.
Moertel, C. G., Rubin, J., & Kvols, L. K. (1989). Therapy of metastatic carcinoid tumor and the malignant carcinoid syndrome with recombinant leukocyte A interferon. Journal of Clinical Oncology, 7(7), 865–868.
Öberg, K., Norheim, I., Lind, E., et al. (1986). Treatment of malignant carcinoid tumors with human leukocyte interferon: Long-term results. Cancer Treatment Reports, 70(11), 1297–1304.
Dirix, L. Y., Vermeulen, P. B., Fierens, H., et al. (1996). Long-term results of continuous treatment with recombinant interferon-alpha in patients with metastatic carcinoid tumors—an antiangiogenic effect? Anti-Cancer Drugs, 7(2), 175–181.
Rinke, A., Muller, H. H., Schade-Brittinger, C., et al. (2009). Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. Journal of Clinical Oncology, 27(28), 4656–4663.
Moertel, C. G., Lefkopoulo, M., Lipsitz, S., et al. (1992). Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. The New England Journal of Medicine, 326(8), 519–523.
Kouvaraki, M. A., Ajani, J. A., Hoff, P., et al. (2004). Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. Journal of Clinical Oncology, 22(23), 4762–4771.
Sun, W., Lipsitz, S., Catalano, P., et al. (2005). Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. Journal of Clinical Oncology, 23(22), 4897–4904.
Öberg, K., & Jelic, S. (2009). Neuroendocrine gastroenteropancreatic tumors: ESMO clinical recommendation for diagnosis, treatment and follow-up. Annals of Oncology, 20(Suppl 4), 150–153.
Öberg, K., Kvols, L., Caplin, M., et al. (2004). Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Annals of Oncology, 15(6), 966–973.
Capdevila, J., & Salazar, R. (2009). Molecular targeted therapies in the treatment of gastroenteropancreatic neuroendocrine tumors. Target Oncology, 4(4), 287–296.
Wullschleger, S., Loewith, R., & Hall, M. N. (2006). TOR signaling in growth and metabolism. Cell, 124(3), 471–484.
Meric-Bernstam, F., & Gonzalez-Angulo, A. M. (2009). Targeting the mTOR signaling network for cancer therapy. Journal of Clinical Oncology, 27(13), 2278–2287.
Arcaro, A., & Guerreiro, A. S. (2007). The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Current Genomics, 8(5), 271–306.
Yuan, R., Kay, A., Berg, W. J., et al. (2009). Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. Journal of Hematology and Oncology, 2, 45.
Vignot, S., Faivre, S., Aguirre, D., et al. (2005). mTOR-targeted therapy of cancer with rapamycin derivatives. Annals of Oncology, 16(4), 525–537.
Franke, T. F., Hornik, C. P., Segev, L., et al. (2003). PI3K/Akt and apoptosis: size matters. Oncogene, 22(56), 8983–8998.
Paez, J., & Sellers, W. R. (2003). PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treatment and Research, 115, 145–167.
LoPiccolo, J., Blumenthal, G. M., Bernstein, W. B., et al. (2008). Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resistance Updates, 11(1–2), 32–50.
von Wichert, G., Jehle, P. M., Hoeflich, A., et al. (2000). Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Research, 60(16), 4573–4581.
Van Gompel, J. J., & Chen, H. (2004). Insulin-like growth factor 1 signaling in human gastrointestinal carcinoid tumor cells. Surgery, 136(6), 1297–1302.
Zatelli, M. C., Minoia, M., Martini, C., et al. (2010). Everolimus as a new potential antiproliferative agent in aggressive human bronchial carcinoids. Endocrine-Related Cancer, 17(3), 719–729.
Tee, A. R., Fingar, D. C., Manning, B. D., et al. (2002). Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proceedings of the National Academy of Sciences of the United States of America, 99(21), 13571–13576.
Verhoef, S., van Diemen-Steenvoorde, R., Akkersdijk, W. L., et al. (1999). Malignant pancreatic tumour within the spectrum of tuberous sclerosis complex in childhood. European Journal of Pediatrics, 158(4), 284–287.
Eledrisi, M. S., Stuart, C. A., & Alshanti, M. (2002). Insulinoma in a patient with tuberous sclerosis: Is there an association? Endocrine Practice, 8(2), 109–112.
Dworakowska, D., & Grossman, A. B. (2009). Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocrine-Related Cancer, 16(1), 45–58.
Hattori, S., Maekawa, M., & Nakamura, S. (1992). Identification of neurofibromatosis type I gene product as an insoluble GTPase-activating protein toward ras p21. Oncogene, 7(3), 481–485.
Johannessen, C. M., Reczek, E. E., James, M. F., et al. (2005). The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proceedings of the National Academy of Sciences of the United States of America, 102(24), 8573–8578.
Blansfield, J. A., Choyke, L., Morita, S. Y., et al. (2007). Clinical, genetic and radiographic analysis of 108 patients with von Hippel–Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery, 142(6), 814–818. discussion 818 e1–2.
Forsythe, J. A., Jiang, B. H., Iyer, N. V., et al. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and Cellular Biology, 16(9), 4604–4613.
Woodward, E. R., & Maher, E. R. (2006). Von Hippel–Lindau disease and endocrine tumour susceptibility. Endocrine-Related Cancer, 13(2), 415–425.
Thakker, R. V. (2010). Multiple endocrine neoplasia type 1 (MEN1). Best Practice & Research. Clinical Endocrinology & Metabolism, 24(3), 355–370.
Debelenko, L. V., Zhuang, Z., Emmert-Buck, M. R., et al. (1997). Allelic deletions on chromosome 11q13 in multiple endocrine neoplasia type 1-associated and sporadic gastrinomas and pancreatic endocrine tumors. Cancer Research, 57(11), 2238–2243.
Jakobovitz, O., Nass, D., DeMarco, L., et al. (1996). Carcinoid tumors frequently display genetic abnormalities involving chromosome 11. The Journal of Clinical Endocrinology and Metabolism, 81(9), 3164–3167.
Öberg, K. (2009). Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors). Current Opinion in Endocrinology, Diabetes and Obesity, 16(1), 72–78.
Wang, E. H., Ebrahimi, S. A., Wu, A. Y., et al. (1998). Mutation of the MENIN gene in sporadic pancreatic endocrine tumors. Cancer Research, 58(19), 4417–4420.
Missiaglia, E., Dalai, I., Barbi, S., et al. (2010). Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. Journal of Clinical Oncology, 28(2), 245–255.
Temsirolimus (Torisel®) (2010). Full prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022088s008lbl.pdf. Accessed 13 Oct 2010.
Durán, I., Kortmansky, J., Singh, D., et al. (2006). A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. British Journal of Cancer, 95(9), 1148–1154.
Everolimus (Afinitor®) (2010). Full prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022334s004lbl.pdf. Accessed 13 Oct 2010.
Zitzmann, K., De Toni, E. N., Brand, S., et al. (2007). The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology, 85(1), 54–60.
Tabernero, J., Rojo, F., Calvo, E., et al. (2008). Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. Journal of Clinical Oncology, 26(10), 1603–1610.
Yao, J. C., Phan, A. T., Chang, D. Z., et al. (2008). Efficacy of RAD001 (Everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. Journal of Clinical Oncology, 26(26), 4311–4318.
Yao, J. C., Lombard-Bohas, C., Baudin, E., et al. (2010). Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. Journal of Clinical Oncology, 28(1), 69–76.
Pavel, M., Hainsworth, J.D., Baudin, E., et al. (2010). A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus plus octreotide LAR vs ersus placebo plus octreotide LAR in patients with advanced neuroendocrine tumors (NET) (RADIANT-2). In: 35th European Medical Oncology Society; 2010; Milan, Italy.
Yao, J.C., Shah, M.H., Ito, T., et al. (2010). A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumors (PNET) (RADIANT-3). In: 35th European Society Medical Oncology; 2010; Milan, Italy
Acknowledgements
The author acknowledges Dr. Ximena Alvira from HealthCo, SL (Madrid, Spain) for her assistance in the preparation of this manuscript and Pfizer Spain for the financial support of medical writing services.
Conflicts of interest
The authors declare that they do not have any conflict of interest that may inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capdevila, J., Salazar, R., Halperín, I. et al. Innovations therapy: mammalian target of rapamycin (mTOR) inhibitors for the treatment of neuroendocrine tumors. Cancer Metastasis Rev 30 (Suppl 1), 27–34 (2011). https://doi.org/10.1007/s10555-011-9290-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9290-3