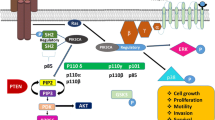
Abstract
The phosphatidyl inositol 3-kinase (PI3K)/Akt pathway mediates the effects of a variety of extracellular signals in a number of cellular processes including cell growth, proliferation, and survival. The alteration of integrants of this pathway through mutation of its coding genes increases the activation status of the signaling and can thus lead to cellular transformation. The frequent dysregulation of the PI3K/Akt pathway in breast cancer (BC) and the mediation of this pathway in different processes characteristically implicated in tumorigenesis have attracted the interest of this pathway in BC; however, a more comprehensive understanding of the signaling intricacies is necessary to develop clinical applications of the modulation of this pathway in this pathology. We review a series of experiments examining the contribution of alteration of integrants of this signaling network to human BC and we make an update of the information about the effect of the modulation of this pathway in this cancer.

Similar content being viewed by others
References
Dillon, R. L., White, D. E., & Muller, W. J. (2007). The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene, 26(9), 1338–1345.
Chalhoub, N., & Baker, S. J. (2009). PTEN and the PI3-kinase pathway in cancer. Annual Review of Patholology, 4, 127–150.
Chitnis, M. M., et al. (2008). The type 1 insulin-like growth factor receptor pathway. Clinical Cancer Research, 14(20), 6364–6370.
Dayanir, V., et al. (2001). Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. Journal of Biological Chemistry, 276(21), 17686–17692.
Dillon, R. L., et al. (2009). Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Research, 69(12), 5057–5064.
Ma, W., & Quirion, R. (2005). The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opinion on Therapeutic Targets, 9(4), 699–713.
Ito, K., Bernardi, R., & Pandolfi, P. P. (2009). A novel signaling network as a critical rheostat for the biology and maintenance of the normal stem cell and the cancer-initiating cell. Current Opinion in Genetics and Development, 19(1), 51–59.
Hirsch, E., et al. (2008). Taming the PI3K team to hold inflammation and cancer at bay. Pharmacology and Therapeutics, 118(2), 192–205.
Thiery, J. P., et al. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139(5), 871–890.
Yilmaz, M., & Christofori, G. (2009). EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Reviews, 28(1–2), 15–33.
Xia, C., et al. (2006). Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. Journal of Cellular Physiology, 209(1), 56–66.
Liang, Z., et al. (2007). CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochemical and Biophysical Research Communications, 359(3), 716–722.
Graupera, M., et al. (2008). Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature, 453(7195), 662–666.
Chin, Y. R., & Toker, A. (2009). Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal, 21(4), 470–476.
S. Loi, B.H.-K., F. Lallemand, L. Pusztai, A. Bardelli, C. Gillett, P. Ellis, M. J. Piccart-Gebhart, W. A. Phillips, G. A. McArthur, C. Sotiriou (2009) Correlation of PIK3CA mutation-associated gene expression signature (PIK3CA-GS) with deactivation of the PI3K pathway and with prognosis within the luminal-B ER + breast cancers. In ASCO. Journal of Clinical Oncology 27:15s (suppl; abstr 533)
Noh, W. C., et al. (2008). Activation of the mTOR signaling pathway in breast cancer and its correlation with the clinicopathologic variables. Breast Cancer Research and Treatment, 110(3), 477–483.
Lopez-Knowles, E., et al. (2010). PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. International Journal of Cancer, 126(5), 1121–1131.
Aleskandarany, M. A., et al. (2010). PIK3CA expression in invasive breast cancer: a biomarker of poor prognosis. Breast Cancer Research and Treatment, 122(1), 45–53.
Marty, B., et al. (2008). Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells. Breast Cancer Research, 10(6), R101.
Aleskandarany, M. A., et al. (2009). PIK3CA expression in invasive breast cancer: a biomarker of poor prognosis. Breast Cancer Research and Treatment., 122(1), 45–53.
Park, S. S., & Kim, S. W. (2007). Activated Akt signaling pathway in invasive ductal carcinoma of the breast: correlation with HER2 overexpression. Oncology Reports, 18(1), 139–143.
Wu, Y., et al. (2008). Clinical significance of Akt and HER2/neu overexpression in African-American and Latina women with breast cancer. Breast Cancer Research, 10(1), R3.
Tokunaga, E., et al. (2006). Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. International Journal of Cancer, 118(2), 284–289.
Mottolese M, N.F., Di Benedetto A, Melucci E, Sperduti I, Perracchio L, Buglioni S, Vici P, Nisticò C, Pinnarò P, Fabi A, Bria E Regina Elena (2009) Identification of an Adverse Biologic Profile in Cyclophosphamide/Metotrexate/5-Fluorouracil Treated Early Stage Breast Cancer Patients by Immunohistochemical Analysis of PI3K/p-Akt Pathway Alterations. In San Antonio Breast Cancer Meeting.
Kirkegaard, T., et al. (2005). AKT activation predicts outcome in breast cancer patients treated with tamoxifen. Journal of Pathology, 207(2), 139–146.
Carpten, J. D., et al. (2007). A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature, 448(7152), 439–444.
Saal, L. H., et al. (2005). PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Research, 65(7), 2554–2559.
Kalinsky, K., et al. (2009). PIK3CA mutation associates with improved outcome in breast cancer. Clinical Cancer Research, 15(16), 5049–5059.
Berns, K., et al. (2007). A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell, 12(4), 395–402.
Stemke-Hale, K., et al. (2008). An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Research, 68(15), 6084–6091.
Perez-Tenorio, G., et al. (2007). PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clinical Cancer Research, 13(12), 3577–3584.
Oda, K., et al. (2005). High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Research, 65(23), 10669–10673.
Li, H., et al. (2009). PIK3CA mutations mostly begin to develop in ductal carcinoma of the breast. Experimental and Molecular Pathology, 88(1), 150–155.
Bose, S., et al. (2002). Reduced expression of PTEN correlates with breast cancer progression. Human Pathology, 33(4), 405–409.
Dunlap, J., et al. (2010). Phosphatidylinositol–3-kinase and AKT1 mutations occur early in breast carcinoma. Breast Cancer Research and Treatment, 120(2), 409–418.
Sakr R, C.S., Wynveen C, Barbashina V, Arroyo C, Morrogh M, Heguy A, Olvera N, Rosen N, Morrow M, King T (2009) Reduced/Absent PTEN Expression Is More Common in HER2 Non-Amplified DCIS. In San Antonio Breast Cancer Meeting
Dupont J, L.A., Knoop A, Ewertz M, Barrett CJ, Hackl W, Bandaru R, Crowell T, Rheinhardt J, Liu W, Gardner H (2009) PIK3CA Mutations Can Be Acquired during Tumor Progression in Breast Cancer. In San Antonio Breast Cancer Meeting
Oda, K., et al. (2008). PIK3CA cooperates with other phosphatidylinositol 3’-kinase pathway mutations to effect oncogenic transformation. Cancer Research, 68(19), 8127–8136.
Renner, O., et al. (2008). Activation of phosphatidylinositol 3-kinase by membrane localization of p110alpha predisposes mammary glands to neoplastic transformation. Cancer Research, 68(23), 9643–9653.
Lai, Y. L., et al. (2008). PIK3CA exon 20 mutation is independently associated with a poor prognosis in breast cancer patients. Annals of Surgical Oncology, 15(4), 1064–1069.
Lerma, E., et al. (2008). Exon 20 PIK3CA mutations decreases survival in aggressive (HER-2 positive) breast carcinomas. Virchows Archiv, 453(2), 133–139.
Maruyama, N., et al. (2007). Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clinical Cancer Research, 13(2 Pt 1), 408–414.
Li, S. Y., et al. (2006). PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Research and Treatment, 96(1), 91–95.
Bachman, K. E., et al. (2004). The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biology & Therapy, 3(8), 772–775.
Campbell, I. G., et al. (2004). Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Research, 64(21), 7678–7681.
Levine, D. A., et al. (2005). Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clinical Cancer Research, 11(8), 2875–2878.
Bozhanov, S.S., et al (2010) Alterations in p53, BRCA1, ATM, PIK3CA, and HER2 genes and their effect in modifying clinicopathological characteristics and overall survival of Bulgarian patients with breast cancer. Journal of Cancer Research and Clinical Oncology, 136(11), 1657–1669.
Vitolo, M. I., et al. (2009). Deletion of PTEN promotes tumorigenic signaling, resistance to anoikis, and altered response to chemotherapeutic agents in human mammary epithelial cells. Cancer Research, 69(21), 8275–8283.
Eng, C. (2003). PTEN: one gene, many syndromes. Human Mutation, 22(3), 183–198.
Chung, M. J., et al. (2004). Inactivation of the PTEN gene protein product is associated with the invasiveness and metastasis, but not angiogenesis, of breast cancer. Pathology International, 54(1), 10–15.
Depowski, P. L., Rosenthal, S. I., & Ross, J. S. (2001). Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Modern Pathology, 14(7), 672–676.
Perren, A., et al. (1999). Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. American Journal of Pathology, 155(4), 1253–1260.
Tsutsui, S., et al. (2005). Reduced expression of PTEN protein and its prognostic implications in invasive ductal carcinoma of the breast. Oncology, 68(4–6), 398–404.
Lee, J. S., et al. (2004). Reduced PTEN expression is associated with poor outcome and angiogenesis in invasive ductal carcinoma of the breast. Applied Immunohistochemistry & Molecular Morphology, 12(3), 205–210.
Shoman, N., et al. (2005). Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Modern Pathology, 18(2), 250–259.
Zhu, L., Loo, W. T., & Louis, W. C. (2007). PTEN and VEGF: possible predictors for sentinel lymph node micro-metastasis in breast cancer. Biomedicine & Pharmacotherapy, 61(9), 558–561.
Panigrahi, A. R., et al. (2004). The role of PTEN and its signalling pathways, including AKT, in breast cancer; an assessment of relationships with other prognostic factors and with outcome. Journal of Pathology, 204(1), 93–100.
Saal, L. H., et al. (2007). Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proceedings of the National Academy of Sciences of the United States of America, 104(18), 7564–7569.
Capodanno, A., et al. (2009). Dysregulated PI3K/Akt/PTEN pathway is a marker of a short disease-free survival in node-negative breast carcinoma. Human Pathology, 40(10), 1408–1417.
Brugge, J., Hung, M. C., & Mills, G. B. (2007). A new mutational AKTivation in the PI3K pathway. Cancer Cell, 12(2), 104–107.
Loi, S., et al (2010) PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10208–10213.
Knuefermann, C., et al. (2003). HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene, 22(21), 3205–3212.
Liedtke, C., et al. (2008). PIK3CA-activating mutations and chemotherapy sensitivity in stage II–III breast cancer. Breast Cancer Research, 10(2), R27.
Yang, S. X., et al. (2010). Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. Journal of Clinical Oncology, 28(18), 2974–2981.
Yamnik, R. L., & Holz, M. K. (2009). mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Letters, 584(1), 124–128.
Perez-Tenorio, G., & Stal, O. (2002). Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. British Journal of Cancer, 86(4), 540–545.
Tokunaga, E., et al. (2006). The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. European Journal of Cancer, 42(5), 629–635.
Miller TW, F.E., Gonzalez-Angulo AM, Hennessy BT, Mills GB, McKinley ET, Manning HC, Arteaga CL (2009) Resistance to endocrine therapy in estrogen receptor-positive (ER+) breast cancer is dependent upon phosphatidylinositol-3 kinase (PI3K) signaling. In San Antonio Breast Cancer Meeting
Stal, O., et al. (2003). Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Research, 5(2), R37–R44.
Miller, T. W., et al. (2010). Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. Journal of Clinical Investigation, 120(7), 2406–2413.
Eichhorn, P. J., et al. (2008). Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Research, 68(22), 9221–9230.
She, S. C., Ye, Q., Lobo, J., Haskell, K. M., Leander, K. R., DeFeo-Jones, D., et al. (2009). Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. Cancer Research, 69, 3061.
Speicher TJ, H.W., Lipton A. (2009) Synergistic Growth Inhibition with a PI3 Kinase/mTOR Inhibitor Plus Lapatinib. In San Antonio Breast Cancer Meeting.
Nagata, Y., et al. (2004). PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell, 6(2), 117–127.
Yao, E., et al. (2009). Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clinical Cancer Research, 15(12), 4147–4156.
Wang, S. E., et al. (2008). Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Molecular and Cellular Biology, 28(18), 5605–5620.
O’Brien, N. A., et al. (2010). Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Molecular Cancer Therapeutics, 9(6), 1489–1502.
Kataoka, Y., et al. (2009). Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Annals of Oncology, 21(2), 255–262.
Vorkas PA, A.S., Poumpouridou N, Kroupis C, Mavroudis D, Stathopoulos E, Georgoulias V, Lianidou ES (2009) PI3K Pathway Activity and Response to First-Line Chemotherapy in Combination with Trastuzumab in Patients with HER2-Positive Metastatic Breast Cancer. In San Antonio Breast Cancer Meeting.
Schnell, C. R., et al. (2008). Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Research, 68(16), 6598–6607.
Courtney, K. D., Corcoran, R. B., & Engelman, J. A. (2010). The PI3K pathway as drug target in human cancer. Journal of Clinical Oncology, 28(6), 1075–1083.
O’Reilly, K. E., et al. (2006). mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Research, 66(3), 1500–1508.
Carracedo, A., et al. (2008). Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. Journal of Clinical Investigation, 118(9), 3065–3074.
Engelman, J. A., et al. (2008). Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nature Medicine, 14(12), 1351–1356.
Bertrand, F. E., et al. (2006). Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia, 20(7), 1254–1260.
Sharon Barr, S.R., Elizabeth Buck, David Epstein, Mark Miglarese (2010) Co-targeting mTOR and IGF-1R/IR results in synergistic activity against a broad array of tumor cell lines, independent of KRAS mutation status. In AACR 101st Annual Meeting.
Serra, V., et al. (2008). NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Research, 68(19), 8022–8030.
She, Q. B., et al. (2008). Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE, 3(8), e3065.
Faber, A. C., et al. (2009). Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proceedings of the National Academy of Sciences of the United States of America, 106(46), 19503–19508.
Sos, M. L., et al. (2009). Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proceedings of the National Academy of Sciences of the United States of America, 106(43), 18351–18356.
Hoeflich, K. P., et al. (2009). In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clinical Cancer Research, 15(14), 4649–4664.
Torbett, N. E., et al. (2008). A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. Biochemical Journal, 415(1), 97–110.
F. Janku, A.M.T., I. Garrido- Laguna, D. S. Hong, A. Naing, G. S. Falchook, J. J. Wheler, S. Fu, S. A. Piha-Paul, R. Kurzrock (2010) PIK3CA, KRAS, and BRAF mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. In ASCO 2010 Meeting. Chicago: Journal of Clinical Oncology
Ma, W. W., et al. (2009). [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. Journal of Clinical Oncology, 27(16), 2697–2704.
Sabine, V. S., et al. (2010). Gene expression profiling of response to mTOR inhibitor everolimus in pre-operatively treated post-menopausal women with oestrogen receptor-positive breast cancer. Breast Cancer Research and Treatment, 122(2), 419–428.
Ozbay, T., et al. (2009). In vitro evaluation of pan-PI3-kinase inhibitor SF1126 in trastuzumab-sensitive and trastuzumab-resistant HER2-over-expressing breast cancer cells. Cancer Chemotherapy and Pharmacology, 65(4), 697–706.
Howes, A. L., et al. (2007). The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Molecular Cancer Therapeutics, 6(9), 2505–2514.
Garlich, J. R., et al. (2008). A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Research, 68(1), 206–215.
H. Burris, J.R., S. Sharma, R. S. Herbst, J. Tabernero, J. R. Infante, A. Silva, D. Demanse, W. Hackl, J. Baselga (2010) First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. In ASCO 2010 Meeting. Journal of Clinical Oncology
D. Sarker, R.K., K. E. Mazina, J. A. Ware, Y. Yan, M. Dresser, M. K. Derynck and J. De-Bono (2009) A phase I study evaluating the pharmacokinetics (PK) and pharmacodynamics (PD) of the oral pan-phosphoinositide-3 kinase (PI3K) inhibitor GDC-0941. In ASCO. Journal of Clinical Oncology
J. Baselga, M.J.D.J., J. Rodon, H. A. Burris III, D. C. Birle, S. S. De Buck, D. Demanse, Q. C. Ru, M. Goldbrunner, J. C. Bendell (2010) A first-in-human phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. In ASCO 2010 Meeting. Journal of Clinical Oncology
Rexer, B. N., Ghosh, R., & Arteaga, C. L. (2009). Inhibition of PI3K and MEK: it is all about combinations and biomarkers. Clinical Cancer Research, 15(14), 4518–4520.
Meric-Bernstam, F., & Gonzalez-Angulo, A. M. (2009). Targeting the mTOR signaling network for cancer therapy. Journal of Clinical Oncology, 27(13), 2278–2287.
Ellard, S. L., et al. (2009). Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. Journal of Clinical Oncology, 27(27), 4536–4541.
Baselga, J., et al. (2009). Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. Journal of Clinical Oncology, 27(16), 2630–2637.
Chan, S., et al. (2005). Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. Journal of Clinical Oncology, 23(23), 5314–5322.
Chow LWC, S.Y., Jassem J, Baselga J, Hayes DF, Wolff AC, Hachemi S, Cincotta M, Yu BW, Kong S, Moore L (2006) Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer. In San Antonio Breast Cancer Meeting. Breast Cancer Research and Treatment
Chen CS, C.M., Imaoka RT, Svahn T, Guardino AE (2009) A Phase I Pilot Study of the Oral mTOR Inhibitor RAD001 in Combination with Capecitabine for Metastatic Breast Cancer. In San Antonio Breast Cancer Meeting.
Cardoso F, D.V., Campone M, Massacesi C, Manlius C, Thevenaz P, Zhang Y, Jerusalem G, Sahmoud T, Andre F, Gianni L (2009) Everolimus (Afinitor®), Trastuzumab (H), and Paclitaxel (P) or Vinorelbine (V) in the Treatment of HER-2+ Metastatic Breast Cancer (MBC) Patients (Pts) Pre-Treated with Lapatinib-Containing Therapy: Pooled Analysis of 2 Phase I Studies. In San Antonio Breast Cancer Meeting.
G. H. Jerusalem, V.D., F. Cardoso, J. Bergh, A. Fasolo, A. Rorive, C. Manlius, I. Pylvaenaeinen, T. Sahmoud, L. Gianni (2008) Multicenter phase I clinical trial of daily and weekly RAD001 in combination with vinorelbine and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab. In ASCO Annual Meeting. Journal of Clinical Oncology
M. Campone, R.O.R., S. Hurvitz, M. Naughton, C. Manlius, L. Vittori, P. Mukhopadhyay, C. Massacesi, T. Sahmoud, F. André (2009) Everolimus plus weekly paclitaxel and trastuzumab in patients (pts) with HER-2+ metastatic breast cancer (MBC) with prior resistance to trastuzumab: a phase I clinical trial. In San Antonio Breast Cancer Meeting.
P. H. Morrow, G.M.W., D. J. Booser, J. A. Moore, P. R. Flores, I. E. Krop, E. P. Winer, G. N. Hortobagyi, D. Yu, F. J. Esteva (2010) Phase I/II trial of everolimus (RAD001) and trastuzumab in patients with trastuzumab-resistant, HER2-overexpressing breast cancer. In ASCO 2010 Meeting. Journal of Clinical Oncology
F. Dalenc, M.C., P. Hupperets, R. O’Regan, C. Manlius, L. Vittori, P. Mukhopadhyay, C. Massacesi, T. Sahmoud, F. Andre (2010) Everolimus in combination with weekly paclitaxel and trastuzumab in patients (pts) with HER2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab and taxanes: A multicenter phase II clinical trial. In ASCO 2010 Meeting. Journal of Clinical Oncology
D. Yardley, M.S., I. Ray-Coquard, B. Melichar, L. Hart, V. Dieras, M. Barve, A. Melnyk, A. Richard, D. Dorer, C. Turner, P. Dodion (2009) Ridaforolimus (AP23573; MK-8669) in combination with trastuzumab for patients with HER2-positive trastuzumab-refractory metastatic breast cancer: a multicenter phase 2 clinical trial. In San Antonio Breast Cancer Meeting.
Acknowledgments
TRANSBIG traineeship programme to Carlos A. Castaneda.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castaneda, C.A., Cortes-Funes, H., Gomez, H.L. et al. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev 29, 751–759 (2010). https://doi.org/10.1007/s10555-010-9261-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-010-9261-0