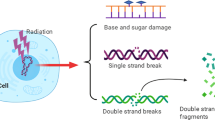
Abstract
Major technical advances in radiotherapy, including IMRT and image-guided radiotherapy, have allowed for improved physical precision and increased dose delivery to the tumor, with better sparing of surrounding normal tissue. The development of inhibitors of the sensing and repair of DNA double-strand breaks (DSBs) is exciting and could be combined with precise radiotherapy targeting to improve local control following radiotherapy. However, caution must be exercised in order that DSB inhibitors are combined with radiotherapy in such a manner as to preserve the therapeutic ratio by exploiting repair deficiencies in malignant cells over that of normal cells. In this review, we discuss the rationale and current approaches to targeting DSB sensing and repair pathways in combined modality with radiotherapy. We also describe potential biomarkers that could be useful in detecting functional inhibition of DSB repair in a patient’s tissues during clinical radiotherapy trials. Finally, we examine a number of issues relating to the use of DSB-inhibiting molecular agents and radiotherapy in the context of the tumor microenvironment, effects on normal tissues and the optimal timing and duration of the agent in relation to fractionated radiotherapy.





Similar content being viewed by others
References
Helleday, T., Petermann, E., Lundin, C., Hodgson, B., & Sharma, R. A. (2008). DNA repair pathways as targets for cancer therapy. Nature Reviews Cancer, 8, 193–204.
Bristow, R. G., & Harrington, L. (2005). Genetic instability and DNA repair. In I. F. Tannock, R. P. Hill, R. G. Bristow, & L. Harrington (Eds.) The basic science of oncology ((pp. 77–99)4th ed.). New York: McGraw-Hill.
Faulhaber, O., & Bristow, R. G. (2005). Basis of cell kill following clinical radiotherapy. In M. Sluyser (Ed.) Application of apoptosis to cancer treatment, vol. 7 (pp. 293–320). Amsterdam: Kluwer–Springer.
Fernet, M., & Hall, J. (2004). Genetic biomarkers of therapeutic radiation sensitivity. DNA Repair (Amst), 3(8–9), 1237–1243.
Andreassen, C. N., Alsner, J., Overgaard, M., Sorensen, F. B., & Overgaard, J. (2006). Risk of radiation-induced subcutaneous fibrosis in relation to single nucleotide polymorphisms in TGFB1, SOD2, XRCC1, XRCC3, APEX and ATM–a study based on DNA from formalin fixed paraffin embedded tissue samples. International Journal of Radiation Biology, 82(8), 577–586.
Olive, P. L., Vikse, C. M., & Durand, R. E. (1994). Hypoxic fractions measured in murine tumors and normal tissues using the comet assay. International Journal of Radiation Oncology, Biology, Physics, 29(3), 487–491.
Bristow, R. G., Ozcelik, H., Jalali, F., Chan, N., & Vesprini, D. (2007). Homologous recombination and prostate cancer: A model for novel DNA repair targets and therapies. Radiotherapy and Oncology, 83(3), 220–230.
Choudhury, A., Cuddihy, A., & Bristow, R. G. (2006). Radiation and new molecular agents part I: Targeting ATM-ATR checkpoints, DNA repair, and the proteasome. Seminars on Radiattion Oncology, 16(1), 51–58.
Olive, P. L. (1999). DNA damage and repair in individual cells: Applications of the comet assay in radiobiology. International Journal of Radiation Biology, 75(4), 395–405.
Bartek, J., & Lukas, J. (2007). DNA damage checkpoints: From initiation to recovery or adaptation. Current Opinion in Cell Biology, 19(2), 238–245.
Jeggo, P. A., & Lobrich, M. (2007). DNA double-strand breaks: Their cellular and clinical impact? Oncogene, 26(56), 7717–7719.
Helleday, T., Lo, J., van Gent, D. C., & Engelward, B. P. (2007). DNA double-strand break repair: From mechanistic understanding to cancer treatment. DNA Repair (Amst), 6(7), 923–935.
O’Connor, M. J., Martin, N. M., & Smith, G. C. (2007). Targeted cancer therapies based on the inhibition of DNA strand break repair. Oncogene, 26(56), 7816–7824.
Yu, T., MacPhail, S. H., Banath, J. P., Klokov, D., & Olive, P. L. (2006). Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA Repair (Amst), 5(8), 935–946.
Cuddihy, A. R., & Bristow, R. G. (2004). The p53 protein family and radiation sensitivity: Yes or no? Cancer and Metastasis Reviews, 23(3–4), 237–257.
Bucher, N., & Britten, C. D. (2008). G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. British Journal of Cancer, 98(3), 523–528.
Harrington, K., Jankowska, P., & Hingorani, M. (2007). Molecular biology for the radiation oncologist: The 5Rs of radiobiology meet the hallmarks of cancer. Clinical Oncology, 19(8), 561–571.
Gordon, A. T., & McMillan, T. J. (1997). A role for molecular radiobiology in radiotherapy? Clinical Oncology, 9(2), 70–78.
Bindra, R. S., Crosby, M. E., & Glazer, P. M. (2007). Regulation of DNA repair in hypoxic cancer cells. Cancer and Metastasis Reviews, 26(2), 249–260.
Chan, N., Koritzinsky, M., Zhao, H., Bindra, R., Glazer, P. M., Powell, S., et al. (2008). Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Research, 68(2), 605–614.
Bristow, R. G., & Hill, R. P. (2008). Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nature Reviews Cancer, 8, 180–192.
Elshaikh, M., Ljungman, M., Ten Haken, R., & Lichter, A. S. (2006). Advances in radiation oncology. Annual Review of Medicine, 57, 19–31.
Bentzen, S. M., & Trotti, A. (2007). Evaluation of early and late toxicities in chemoradiation trials. Journal of Clinical Oncology, 25(26), 4096–4103.
Bentzen, S. M. (2006). Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nature Reviews Cancer, 6(9), 702–713.
Hill, R. P., Rodemann, H. P., Hendry, J. H., Roberts, S. A., & Anscher, M. S. (2001). Normal tissue radiobiology: From the laboratory to the clinic. International Journal of Radiation Oncology, Biology, Physics, 49(2), 353–365.
Chen, D. J., & Nirodi, C. S. (2007). The epidermal growth factor receptor: A role in repair of radiation-induced DNA damage. Clinical Cancer Research, 13(22 Pt 1), 6555–6560.
Rochester, M. A., Riedemann, J., Hellawell, G. O., Brewster, S. F., & Macaulay, V. M. (2005). Silencing of the IGF1R gene enhances sensitivity to DNA-damaging agents in both PTEN wild-type and mutant human prostate cancer. Cancer Gene Therapy, 12(1), 90–100.
Dupre, A., Boyer-Chatenet, L., Sattler, R. M., Modi, A. P., Lee, J. H., Nicolette, M. L., et al. (2008). A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nature Chemical Biology, 4(2), 119–125.
Olive, P. L., & Banath, J. P. (2004). Phosphorylation of histone H2AX as a measure of radiosensitivity. International Journal of Radiation Oncology, Biology, Physics, 58(2), 331–335.
Hammond, E. M., Kaufmann, M. R., & Giaccia, A. J. (2007). Oxygen sensing and the DNA-damage response. Current Opinion in Cell Biology, 19(6), 680–684.
Dalton, W. S., & Friend, S. H. (2006). Cancer biomarkers—An invitation to the table. Science, 312(5777), 1165–1168.
Lavin, M. F., Delia, D., & Chessa, L. (2006). ATM and the DNA damage response. Workshop on ataxia-telangiectasia and related syndromes. EMBO Reports, 7(2), 154–160.
Canman, C. E., Lim, D. S., Cimprich, K. A., Taya, Y., Tamai, K., Sakaguchi, K., et al. (1998). Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science, 281(5383), 1677–1679.
Banin, S., Moyal, L., Shieh, S., Taya, Y., Anderson, C. W., Chessa, L., et al. (1998). Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science, 281(5383), 1674–1677.
Hall-Jackson, C. A., Cross, D. A., Morrice, N., & Smythe, C. (1999). ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene, 18(48), 6707–6713.
Collis, S. J., DeWeese, T. L., Jeggo, P. A., & Parker, A. R. (2005). The life and death of DNA-PK. Oncogene, 24(6), 949–961.
Chen, B. P., Chan, D. W., Kobayashi, J., Burma, S., Asaithamby, A., Morotomi-Yano, K., et al. (2005). Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. Journal of Biological Chemistry, 280(15), 14709–14715.
Madhusudan, S., & Middleton, M. R. (2005). The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treatment Reviews, 31(8), 603–617.
Lord, C. J., Garrett, M. D., & Ashworth, A. (2006). Targeting the double-strand DNA break repair pathway as a therapeutic strategy. Clinical Cancer Research, 12(15), 4463–4468.
Hickson, I., Zhao, Y., Richardson, C. J., Green, S. J., Martin, N. M., Orr, A. I., et al. (2004). Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Research, 64(24), 9152–9159.
Chalmers, A. J. (2004). Poly(ADP-ribose) polymerase-1 and ionizing radiation: Sensor, signaller and therapeutic target. Clinical Oncology, 16(1), 29–39.
Schraufstatter, I. U., Hyslop, P. A., Hinshaw, D. B., Spragg, R. G., Sklar, L. A., & Cochrane, C. G. (1986). Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proceedings of the National Academy of Sciences of the United States of America, 83(13), 4908–4912.
Bakkenist, C. J., & Kastan, M. B. (2003). DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature, 421(6922), 499–506.
Bartek, J., & Lukas, J. (2003). DNA repair: Damage alert. Nature, 421(6922), 486–488.
Chan, D. W., Chen, B. P., Prithivirajsingh, S., Kurimasa, A., Story, M. D., Qin, J., et al. (2002). Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes & Development, 16(18), 2333–2338.
Matsuoka, S., Ballif, B. A., Smogorzewska, A., McDonald 3rd, E. R., Hurov, K. E., Luo, J., et al. (2007). ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science, 316(5828), 1160–1166.
Kawamitsu, H., Hoshino, H., Okada, H., Miwa, M., Momoi, H., & Sugimura, T. (1984). Monoclonal antibodies to poly(adenosine diphosphate ribose) recognize different structures. Biochemistry, 23(16), 3771–3777.
Plummer, E. R., Middleton, M. R., Jones, C., Olsen, A., Hickson, I., McHugh, P., et al. (2005). Temozolomide pharmacodynamics in patients with metastatic melanoma: DNA damage and activity of repair enzymes O6-alkylguanine alkyltransferase and poly(ADP-ribose) polymerase-1. Clinical Cancer Research, 11(9), 3402–3409.
Kinders, R. J., Palma, J., Liu, X., Colon-Lopez, M., Luo, Y., Rodriguez, L. E., et al. (2006). Development of a quantitative enzyme immunoassay for measurement of PAR as a pharmacodynamic biomarker of PARP activity. AACR Meeting Abstracts, 2006(2), A5.
Plummer, R., Middleton, M., Wilson, R., Jones, C., Evans, L., Robson, L., et al. (2005). First in human phase I trial of the PARP inhibitor AG-014699 with temozolomide (TMZ) in patients (pts) with advanced solid tumors. Journal of Clinical Oncology, 23(16S), 3065.
Ratnam, K., & Low, J. A. (2007). Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clinical Cancer Research, 13(5), 1383–1388.
Sreekumar, A., Nyati, M. K., Varambally, S., Barrette, T. R., Ghosh, D., Lawrence, T. S., et al. (2001). Profiling of cancer cells using protein microarrays: Discovery of novel radiation-regulated proteins. Cancer Research, 61(20), 7585–7593.
Dainiak, N., Schreyer, S. K., & Albanese, J. (2005). The search for mRNA biomarkers: Global quantification of transcriptional and translational responses to ionising radiation. BJR Supplement, 27, 114–122.
Menard, C., Johann, D., Lowenthal, M., Muanza, T., Sproull, M., Ross, S., et al. (2006). Discovering clinical biomarkers of ionizing radiation exposure with serum proteomic analysis. Cancer Research, 66(3), 1844–1850.
Lukas, C., Bartek, J., & Lukas, J. (2005). Imaging of protein movement induced by chromosomal breakage: Tiny ‘local’ lesions pose great ‘global’ challenges. Chromosoma, 114(3), 146–154.
Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S., & Bonner, W. M. (1998). DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. Journal of Biological Chemistry, 273(10), 5858–5868.
Paull, T. T., Rogakou, E. P., Yamazaki, V., Kirchgessner, C. U., Gellert, M., & Bonner, W. M. (2000). A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Current Biology, 10(15), 886–895.
Essers, J., Houtsmuller, A. B., van Veelen, L., Paulusma, C., Nigg, A. L., Pastink, A., et al. (2002). Nuclear dynamics of RAD52 group homologous recombination proteins in response to DNA damage. EMBO Journal, 21(8), 2030–2037.
Shiloh, Y. (2003). ATM and related protein kinases: Safeguarding genome integrity. Nature Reviews Cancer, 3(3), 155–168.
Lukas, C., Falck, J., Bartkova, J., Bartek, J., & Lukas, J. (2003). Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nature Cell Biology, 5(3), 255–260.
Bekker-Jensen, S., Lukas, C., Kitagawa, R., Melander, F., Kastan, M. B., Bartek, J., et al. (2006). Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. Journal of Cell Biology, 173(2), 195–206.
Haaf, T., Golub, E. I., Reddy, G., Radding, C. M., & Ward, D. C. (1995). Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proceedings of the National Academy of Sciences of the United States of America, 92(6), 2298–2302.
Olive, P. L., Banath, J. P., & Keyes, M. (2008). Residual gammaH2AX after irradiation of human lymphocytes and monocytes in vitro and its relation to late effects after prostate brachytherapy. Radiotherapy and Oncology, 86(3), 336–346.
van Veelen, L. R., Cervelli, T., van de Rakt, M. W., Theil, A. F., Essers, J., & Kanaar, R. (2005). Analysis of ionizing radiation-induced foci of DNA damage repair proteins. Mutation Research, 574(1–2), 22–33.
Sedelnikova, O. A., Rogakou, E. P., Panyutin, I. G., & Bonner, W. M. (2002). Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiation Research, 158(4), 486–492.
Banath, J. P., & Olive, P. L. (2003). Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Research, 63(15), 4347–4350.
Klokov, D., MacPhail, S. M., Banath, J. P., Byrne, J. P., & Olive, P. L. (2006). Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiotherapy and Oncology, 80(2), 223–229.
Rothkamm, K., Balroop, S., Shekhdar, J., Fernie, P., & Goh, V. (2007). Leukocyte DNA damage after multi-detector row CT: A quantitative biomarker of low-level radiation exposure. Radiology, 242(1), 244–251.
Hamasaki, K., Imai, K., Nakachi, K., Takahashi, N., Kodama, Y., & Kusunoki, Y. (2007). Short-term culture and gammaH2AX flow cytometry determine differences in individual radiosensitivity in human peripheral T lymphocytes. Environmental and Molecualr Mutagenesis, 48(1), 38–47.
Tanaka, T., Huang, X., Halicka, H. D., Zhao, H., Traganos, F., Albino, A. P., et al. (2007). Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry A, 71(9), 648–661.
MacPhail, S. H., Banath, J. P., Yu, T. Y., Chu, E. H., Lambur, H., & Olive, P. L. (2003). Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. International Journal of Radiation Biology, 79(5), 351–358.
Olive, P. L. (2004). Detection of DNA damage in individual cells by analysis of histone H2AX phosphorylation. Methods in Cell Biology, 75, 355–373.
Lobrich, M., & Kiefer, J. (2006). Assessing the likelihood of severe side effects in radiotherapy. International Journal of Cancer, 118(11), 2652–2656.
Taneja, N., Davis, M., Choy, J. S., Beckett, M. A., Singh, R., Kron, S. J., et al. (2004). Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. Journal of Biological Chemistry, 279(3), 2273–2280.
Rogakou, E. P., Nieves-Neira, W., Boon, C., Pommier, Y., & Bonner, W. M. (2000). Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. Journal of Biological Chemistry, 275(13), 9390–9395.
Kumaravel, T. S., & Jha, A. N. (2006). Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutation Research, 605(1–2), 7–16.
Dorie, M. J., Kovacs, M. S., Gabalski, E. C., Adam, M., Le, Q. T., Bloch, D. A., et al. (1999). DNA damage measured by the comet assay in head and neck cancer patients treated with tirapazamine. Neoplasia, 1(5), 461–467.
Terris, D. J., Ho, E. Y., Ibrahim, H. Z., Dorie, M. J., Kovacs, M. S., Le, Q. T., et al. (2002). Estimating DNA repair by sequential evaluation of head and neck tumor radiation sensitivity using the comet assay. Archives of Otolaryngology Head & Neck Surgery, 128(6), 698–702.
Francis, R. J., Sharma, S. K., Springer, C., Green, A. J., Hope-Stone, L. D., Sena, L., et al. (2002). A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. British Journal of Cancer, 87(6), 600–607.
Prise, K. M., Ahnstrom, G., Belli, M., Carlsson, J., Frankenberg, D., Kiefer, J., et al. (1998). A review of dsb induction data for varying quality radiations. International Journal of Radiation Biology, 74(2), 173–184.
Bohm, L. (2006). Inhibition of homologous recombination repair with Pentoxifylline targets G2 cells generated by radiotherapy and induces major enhancements of the toxicity of cisplatin and melphalan given after irradiation. Radiation Oncology, 1, 12.
Grem, J. L., Harold, N., Keith, B., Chen, A. P., Kao, V., Takimoto, C. H., et al. (2002). A phase I pharmacologic and pharmacodynamic study of pyrazoloacridine given as a weekly 24-hour continuous intravenous infusion in adult cancer patients. Clinical Cancer Research, 8(7), 2149–2156.
Kumaravel, T. S., & Bristow, R. G. (2005). Detection of genetic instability at HER-2/neu and p53 loci in breast cancer cells using Comet-FISH. Breast Cancer Research and Treatment, 91(1), 89–93.
Bayani, J., & Squire, J. A. (2007). Application and interpretation of FISH in biomarker studies. Cancer Letters, 249(1), 97–109.
Ma, B. B., Bristow, R. G., Kim, J., & Siu, L. L. (2003). Combined-modality treatment of solid tumors using radiotherapy and molecular targeted agents. Journal of Clinical Oncology, 21(14), 2760–2776.
Parulekar, W. R., & Eisenhauer, E. A. (2004). Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: Theory and practice. Journal of National Cancer Institute, 96(13), 990–997.
Deutsch, E., Soria, J. C., & Armand, J. P. (2005). New concepts for phase I trials: Evaluating new drugs combined with radiation therapy. Nature Clinical Practice Oncology, 2(9), 456–465.
Dowlati, A., Haaga, J., Remick, S. C., Spiro, T. P., Gerson, S. L., Liu, L., et al. (2001). Sequential tumor biopsies in early phase clinical trials of anticancer agents for pharmacodynamic evaluation. Clinical Cancer Research, 7(10), 2971–2976.
Agulnik, M., Oza, A. M., Pond, G. R., & Siu, L. L. (2006). Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. Journal of Clinical Oncology, 24(30), 4801–4807.
Helft, P. R., & Daugherty, C. K. (2006). Are we taking without giving in return? The ethics of research-related biopsies and the benefits of clinical trial participation. Journal of Clinical Oncology, 24(30), 4793–4795.
Cannistra, S. A. (2007). Performance of biopsies in clinical research. Journal of Clinical Oncology, 25(11), 1454–1455.
Rockett, J. C., Burczynski, M. E., Fornace, A. J., Herrmann, P. C., Krawetz, S. A., & Dix, D. J. (2004). Surrogate tissue analysis: Monitoring toxicant exposure and health status of inaccessible tissues through the analysis of accessible tissues and cells. Toxicology and Applied Pharmacology, 194(2), 189–199.
Rehman, S., & Jayson, G. C. (2005). Molecular imaging of antiangiogenic agents. Oncologist, 10(2), 92–103.
Cullinane, C., Dorow, D. S., Kansara, M., Conus, N., Binns, D., Hicks, R. J., et al. (2005). An in vivo tumor model exploiting metabolic response as a biomarker for targeted drug development. Cancer Research, 65(21), 9633–9636.
Yang, D. J., Kim, E. E., & Inoue, T. (2006). Targeted molecular imaging in oncology. Annals of Nuclear Medicine, 20(1), 1–11.
Boothman, D. A., Bouvard, I., & Hughes, E. N. (1989). Identification and characterization of X-ray-induced proteins in human cells. Cancer Research, 49(11), 2871–2878.
Einspahr, J. G., Alberts, D. S., Gapstur, S. M., Bostick, R. M., Emerson, S. S., & Gerner, E. W. (1997). Surrogate end-point biomarkers as measures of colon cancer risk and their use in cancer chemoprevention trials. Cancer Epidemiology Biomarkers & Prevention, 6(1), 37–48.
Pepe, M. S., Etzioni, R., Feng, Z., Potter, J. D., Thompson, M. L., Thornquist, M., et al. (2001). Phases of biomarker development for early detection of cancer. Journal of National Cancer Institute, 93(14), 1054–1061.
Sharma, R. A., & Farmer, P. B. (2004). Biological relevance of adduct detection to the chemoprevention of cancer. Clinical Cancer Research, 10(15), 4901–4912.
Hede, K. (2005). NCI’s National Biospecimen Network: Too early or too late? Journal of National Cancer Institute, 97(4), 247–248.
Maruvada, P., & Srivastava, S. (2006). Joint National Cancer Institute-Food and Drug Administration workshop on research strategies, study designs, and statistical approaches to biomarker validation for cancer diagnosis and detection. Cancer Epidemiology Biomarkers & Prevention, 15(6), 1078–1082.
Bartek, J., Bartkova, J., & Lukas, J. (2007). DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene, 26(56), 7773–7779.
Acknowledgements
Work in the author’s laboratories is supported by grants from National Cancer Institute of Canada, the Terry Fox Foundation and the Canadian Institutes of Health Research (PO, RGB). RGB is a Canadian Cancer Society Research Scientist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S.K., Olive, P.L. & Bristow, R.G. Biomarkers for DNA DSB inhibitors and radiotherapy clinical trials. Cancer Metastasis Rev 27, 445–458 (2008). https://doi.org/10.1007/s10555-008-9137-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9137-8