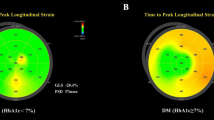
Abstract
Purpose
Speckle tracking echocardiography (STE) can help to identify subclinical features of diabetic cardiomyopathy (DCM). There is, however, significant heterogeneity in the reported strain values in literature. We performed a systematic review and meta-analysis to compare cardiac systolic strain values assessed by 2D-STE in asymptomatic adults with diabetes mellitus (DM) and healthy controls.
Methods
Five databases were searched, and a total of 41 valid studies (6668 individuals with DM and 7218 controls) were included for analysis. Pooled mean in each group and mean difference (MD) for left ventricular global longitudinal strain (LVGLS), LV global circumferential strain (LVGCS), LV global radial strain (LVGRS), LV longitudinal systolic strain rate (LVSR), left atrial reservoir strain (LARS) and right ventricular GLS (RVGLS) were assessed.
Results
Patients with DM had overall 2 units lower LVGLS than healthy subjects 17.5% [16.8, 18.3], vs 19.5 [18.7, 20.4], MD = − 1.96 [− 2.27, − 1.64]. Other strain values were also lower in patients with DM: LVGCS (MD = − 0.89 [− 1.26, − 0.51]); LVGRS (MD = − 5.03 [− 7.18, − 2.87]); LVSR (MD = − 0.06 [− 0.10, − 0.03]); LARS (MD = − 8.41 [− 11.5, − 5.33]); and RVGLS (MD = − 2.41 [− 3.60, − 1.22]). Meta-regression identified higher body mass index (BMI) as the single contributor to worse LVGLS, LVGCS and LVSR. Those with higher Hemoglobulin A1c had worse RVGLS.
Conclusion
Myocardial strains were reduced in whole heart in patients with DM. The largest reduction was observed in LA reservoir strain, followed by RVGLS and LVGLS. Higher BMI in patients with DM is associated with worse LV strain values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is one of the most prevalent chronic diseases in the world [1], and contributes to significant cardiac mortality and morbidity [2, 3]. The risk of heart failure increases at least 2–5 times in patients with DM [2, 3]. Diabetic cardiomyopathy (DCM) is defined as the development of myocardial dysfunction in individuals with DM, independent of coronary artery disease, hypertension, valvular, or congenital heart disease [3]. Although most of the previous studies using conventional echocardiography have emphasized left ventricular (LV) diastolic dysfunction as the earliest and main functional alteration in the course of DCM [4, 5, 6, 7], some recent studies using speckle tracking echocardiography (STE) have reported subclinical systolic dysfunction in adults with DM predates the development of LV diastolic dysfunction [8]. Early detection of subclinical and reversible cardiac dysfunction in patients with DM using STE would lead to treatment, which could prevents subsequent development of heart failure[9].
Investigators have tried to clarify the impact of DM on cardiac mechanics using STE recently [10, 11, 12]. Most of these studies reported impaired global longitudinal strain (GLS) in asymptomatic patients with DM [13, 14, 15, 16]. However, the current data are still conflicting and non-homogeneous. Some studies reported similar GLS between individuals with DM and controls [10, 17, 18, 19]. The measured GLS in patients with DM in some studies are higher than measured GLS in healthy controls of some other studies [20, 21, 22]. In addition, the exact extent of the decrease and alteration of left ventricle GLS (LVGLS) and alteration of strain in other directions (circumferential and radial) in individuals with DM have been less studied [10, 20, 23]. Finally, assessment of left atrial (LA) and right ventricle (RV) mechanics using STE in asymptomatic patients with DM is a new era of interest [12, 24].
Thus, we aimed (1) to conduct a systematic review on the strain values of LV, LA and RV assessing by 2D-STE in asymptomatic adults with DM and healthy controls; (2) to synthesize the information qualitatively; (3) to perform quantitative analysis using meta-analysis to estimate the pooled mean difference (MD) of these strain values in individuals with DM and controls; and (4) to clarify possible sources of variation affecting the strain values by meta-regression analysis.
Methods
Search strategy
We performed a systematic review and meta-analysis following the PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) guideline. Under the guidance of a librarian at the University of Sydney, we searched five databases (MEDLINE, Embase, Scopus, Web of Science and Cochrane central register of controlled trials) for the key terms of “myocardial strain/ LV, RV, LA/function, dysfunction”, “speckle tracking echocardiography, deformation imaging/analysis” and “diabetes mellitus”. The search was limited to human articles published in English and completed on March 30, 2020. Search hedges created are listed in the Online Supplementary Materials (Appendix A). The reference lists of relevant studies were manually searched for any possible additional appropriate study. The study was prospectively registered with the PROSPERO database of systematic reviews (Subclinical systolic dysfunction detected by 2D speckle tracking echocardiography in diabetes mellitus: a systematic review and meta-analysis; CRD42020197825).
Study selection
From these lists, studies were included if the articles reported strain values using 2D-STE in asymptomatic patients with DM and control group. Two independent investigators (S.G and A.G) reviewed and chose studies if the articles met the following criteria: (1) studies reported strain values of LV and/or LA and/or RV in adult patients with DM (type 1 or 2), (2) studies included a control group, 3) were > 18 years of mean age. The definition of each group and exclusion criteria varies with the studies and are shown in Online Supplementary Materials (Online Table S1). If one study had multiple groups of patients or controls, we selected the lower risk group for our meta-analysis to avoid extreme cases. When multiple studies that used the same data set were identified, the largest study was included for assessment and analysis.
Study exclusion
Our exclusion criteria were reduced ejection fraction, presence of known coronary artery disease (CAD), or any structural heart disease. Multiple studies used different methods to exclude CAD patients (e.g. presence of known history or symptoms of CAD, positive non-invasive investigations). Detailed exclusion criteria of each study are shown in Supplementary Table S1. We also excluded studies in which strain was calculated using 3D-STE, Doppler tissue imaging, or cardiac magnetic resonance imaging. In addition, case reports, conference presentations, review articles, editorial, and expert opinions were excluded.
Data collection
All demographic, ultrasound system and software, common clinical characteristics, and strain information were extracted from texts, tables, and graphs and summarized into a standardized extraction sheet. Authors of eligible studies were contacted by e-mail to obtain missing information.
The outcome of interest
In this meta-analysis, our outcomes of interest were LV, LA and RV strains (LVGLS, LV global circumferential strain (LVGCS), LV global radical strain (LVGRS), LV longitudinal systolic strain rate (LVSR), LA reservoir strain, and RVGLS) measured by 2D-STE in adults with DM and control groups.
Statistical analysis
The pooled means and 95% confidence interval (CI) of LVGLS, LVGCS, LVGRS, LVSR, LA reservoir strain, and RVGLS in patients with DM and control groups were computed using random-effects models weighted by inverse variance and are showed in the forest plot. Although our primary outcome was MD, we also calculated standardized mean difference (SMD, also known as Cohen’s D) in each study and pooled to compare the effect sizes among various strains with different normal ranges[25, 26]. SMD = 0.2 is considered as a small effect size, SMD = 0.5 as a ‘medium’ effect size, and SMD = 0.8 as a large effect size [25]. The heterogeneity between studies were assessed by the Cochran Q test and the inconsistency factor (I2). I2 values of 25%, 50%, and 75% corresponded to a low, moderate, and high degree of heterogeneity, respectively. Potential publication bias was assessed using Funnel plots with and without the Duval and Tweedie trim and fill methodology, and the Egger’s test. Meta-regression analysis was performed for variables that were reported in > 50% of studies to assess possible study factors associated with heterogeneity. The beta coefficient and its CIs were derived using the least-mean squares fitting method. Sensitivity analyses were performed to examine the effect of methodological diversity (definition of study groups based on the inclusion of hypertensive patients) on the overall pooled estimates. Statistical analysis was performed using R version 4.0.0 and RStudio version 1.2.5042 (The R Foundation for Statistical Computing, Vienna, Austria) with the “metafor” package. Two-tailed p values were used, and the threshold of statistical significance was 0.05 except for the Egger test, where 0.1 was applied. Based on the EACVI/ASE/Industry taskforce recommendation [27] and to avoid unnecessary confusion, we considered the absolute value of each strain value.
Results
Study selection
Figure 1 shows PRISMA flowchart of our study. Our search strategy revealed 791 results from 5 databases (MEDLINE [n = 121], EMBASE [n = 330], Scopus [n = 40], Web of Science [n = 290], Cochrane central register of controlled trials [n = 10]). Following the removal of 259 duplicates, the titles and abstracts of 532 articles were screened for eligibility. Four hundred and forty-two studies were excluded because of the different study populations and different study designs (no control group, CMR study, Doppler tissue imaging). Ninety full-text articles assessed for eligibility. An additional 49 studies were excluded for the following reasons: no GLS data, Doppler tissue imaging, just 3D-STE results, paediatrics, and patients with coronary artery disease. Finally, 41 valid studies (6668 individuals with DM and 7218 controls) met the selection criteria and were included in this meta-analysis. Thirty-two studies were eligible for LVGLS, 14 for LVGCS, 9 for LVGRS, 13 for LVSR, 7 for LA reservoir strain, and 7 for RVGLS. Articles included were published from 2009 to 2020. Most of the studies used age and gender-matched healthy subjects for the control group. Summary of included studies is shown in Table 1. Further detailed information can be found in online Supplementary Materials (Tables S2 and S3).
PRISMA Flow Chart this flow chart illustrates the selection process for published reports on LV, LA and RV strains (LVGLS, LVGCS, LVGRS, LVSR, LA reservoir strain and RVGLS) measured by 2D-STE in adult with DM and control groups. GLS global longitudinal strain; GCS global circumferential strain; GRS global radial strain; LVSR left ventricular longitudinal systolic strain rate; LV left ventricular; RV right ventricular; DM diabetes mellitus
LV strain in adults with DM vs controls
All LV strain values (GLS, GCS, GRS, and LVSR) were reduced in adults with DM compared to healthy subjects. Patients with DM had significantly lower LVGLS than healthy subjects (17.5% [16.8, 18.3] vs 19.5% [18.7, 20.4]) with MD of − 1.96% [− 2.27, − 1.64]) (Fig. 2 and Table 2). LVGCS, LVGRS, and LVSR were also lower in individuals with DM, but the effect sizes were small (Table 2, Online Figures S1–S3). Although no significant publication bias was identified by the funnel plot with and without Trim and Fill (Online Figures S4–S7) and the Egger’s test (except for LVGCS of controls and LVGRS of patients with DM), there were a high degree of heterogeneity in all LV strain values. Therefore, a univariate meta-regression was performed to find factors that have significant contributions to the heterogeneity (Table 3). It revealed that increasing body mass index (BMI) was associated with worse LVGLS, LVGCS, and LVSR. In addition, studies that used Wall Motion Tracking software [22, 28] had reported significantly lower LVGLS and LVGCS compared to EchoPAC software (β for LVGLS of DM = − 3.79 [− 7.05, − 0.53], p = 0.02; β for LVGCS of DM = − 5.17 [− 10.14, -0.2], p = 0.04).
LA strain in adults with DM vs controls
LA reservoir strain was significantly lower in individuals with DM compared to healthy controls (28.0% [23.9, 32.1] vs 36.5 [34.2, 38.9]) with a large MD of − 8.41 [− 11.5, − 5.33]) (Fig. 3 and Table 2). Egger’s test showed a significant publication bias in patients with DM (p = 0.02). Although a high degree of heterogeneity was identified in adults with DM (I2 = 98.59%), meta-regression could not find any significant contributor to this heterogeneity (Table 3). Funnel plots for LA reservoir strain with and without Trim and Fill in adults with DM and control groups are shown in the online Fig. S8.
RV strain in adults with DM vs controls
RVGLS was also significantly lower in adults with DM (24.1% [23.0, 25.1] vs 26.0 [24.0, 28.0]) with an MD of − 2.41 [− 3.60, − 1.22]) (Fig. 4 and Table 2). Although there was no significant publication bias in the DM group, a high degree of heterogeneity was identified (I2 = 98.89%). We found that female proportion, diastolic blood pressure, and haemoglobin A1c (HbA1C) were the factors linked to this heterogeneity in patients with DM (Table 3). Funnel plots for RVGLS with and without Trim and Fill in individuals with DM and control groups are shown in Fig. S9.
Additional analysis
Sensitivity analyses based on the inclusion or exclusion of hypertensive patients in each study revealed no obvious effects of hypertension on LVGLS (Online Figure S10).
In the present study, in order to evaluate DCM patients, we initially excluded studies that reported strain values in patients with DM and CAD in the main analyses. There were 4 studies [23, 29, 30, 31] that reported LVGLS in patients with DM and CAD. Table 4 summarizes these studies. The pooled mean LVGLS in patients with both DM and CAD was 16.4% [15.2, 17.6] (Online Figure S11), whereas the pooled mean of LVGLS in patients with DM without CAD was 17.8 [17, 18.6].
Discussion
This is the first systematic review and meta-analysis of the pooled difference of cardiac strain values assessed by 2D-STE in 6,668 asymptomatic patients with DM compared to 7218 healthy controls from 41 studies. There were three major findings. First, systolic strain values are significantly reduced in asymptomatic patients with DM, not only in the LV but also in the LA and RV. This confirms that DCM is a global cardiac phenomenon and not merely an LV dysfunction. Second, LA reservoir strain, RVGLS, and LVGLS had large effect sizes with SMD > 0.8. Finally, higher BMI associated with worse LVGLS, LVGRS, and LVSR, whereas higher HbA1c is the main contributor to worse RVGLS in patients with DM.
Our meta-analysis confirmed that subclinical cardiac dysfunction in DCM is not limited to the LV but also seen in the RV and LA. The effect size assessed by SMD was the largest in LA reservoir strain (SMD − 1.2 [− 1.5, − 0.9]), closely followed by RVGLS (− 1.1 [− 1.9, − 0.2]) and LVGLS (− 0.8 [− 1.0, − 0.7]). Larger effect sizes in the LA and RV further support the whole heart dysfunction in DCM. The clear separation of LA reservoir strain between patients with DM (upper limit of 95%CI of 32.1%) and control (lower limit of 95%CI of 34.2%) (Table 2) indicates that LA reservoir strain can be a good diagnostic parameter. Similar separation was observed in LVGLS, where lower limit of LVGLS in control was 18.7% and upper limit of DM was 18.3%. On contrary, the reduction in LVGCS was small by − 0.89% [− 1.26, − 0.51] although it was still statistically significant. The reason for the discrepancy in effect sizes between LVGLS and LVGCS is still unclear [32, 33, 34]. Some studies argued that in early stages of myocardial dysfunction, impairment in longitudinal deformation accompanies by a compensatory increase in circumferential deformation to preserve of gross LVEF [34]. The decline in circumferential deformation only occurs in advanced stages and ultimately results in falling LVEF [35]. However, our meta-analysis showed that asymptomatic patients with DM with normal LVEF have impaired LVGCS and LVGRS in addition to the impaired LVGLS. This corroborates our previous findings in 3D STE, where DCM is at least pan-LV phenomena rather than reduction in a single direction [36]. More studies are warranted to reveal the changes in multiple directions of myocardial strains during the evolvement of DCM.
Our meta-regression showed that BMI is the only significant source of heterogeneity in subclinical LV systolic dysfunction measured by 2D-STE in patients with DM. We confirmed that increasing BMI was associated with worse LVGLS, LVGCS, and LVSR in patients with DM. The impact of obesity on LV function in adults with DM has been investigated in some studies and they were also included in the present meta-analysis [15, 16, 37]. These studies showed that increasing BMI and DM are independent predictors of impaired LV myocardial systolic dysfunction. Ng et al. reported that not only the combination of DM and higher BMI has an additive detrimental effect on LV myocardial function, but also increasing BMI per se is a stronger determinant of impaired LV myocardial function than DM [37]. On the other hand, we found that increasing HbA1c, as the main source of heterogeneity in RVGLS, was significantly associated with worse RVGLS in both adults with DM and control groups.
Little is known that the additive effects of concomitant CAD on myocardial deformation of DCM. Four studies reported strain values in patients with DM and CAD [23, 29, 30, 31] (Table 4). Although from limited number of articles, LV GLS of less than 17 may suggest the possibility of concomitant CAD.
Based on convincing results of EMPA-REG OUTCOME [38] study and other similar trials [39], 2019 ESC Guidelines on DM, pre-DM and cardiovascular diseases (CVD) recommended the use of sodium-glucose co-transporter 2 (SGLT2) inhibitors in patients with T2DM and CVD or at high/very high cardiovascular (CV) risk to reduce CV events. Recent data suggest that these relatively new glucose-lowering drugs can prevent heart failure in patients with DM. However, potential candidates of SGLT2 inhibitors are not clearly defined. Our systematic review and meta-analysis showed that 2D-STE can be helpful in the diagnosis of subclinical DCM in early stages. Therefore, patients with lower strain values in 2D-STE can be potential candidates to treat with SGLT2 inhibitors to prevent and treat subsequent clinical heart failure.
Study limitations
Several factors merit consideration in the interpretation of our results. First, like all meta-analyses, this study is limited by quality in the original studies and publication bias, although we used standard approaches to detect this. In addition, observational studies may be limited by biases in the recruitment process. Second, we have assumed that all the measurements were performed by the experts, but the levels of expertise among individuals who have measured the strain are uncertain. Third, significant heterogeneities among studies were identified. Thus, we performed subsequent meta-regression analyses to explain the sources of the variations. Fourth, this study does not have information about right atrial strain values. Fifth, included studies did not outline information regarding duration of DM in the recruited patients, and therefore could not be assessed for impact on heterogeneity. Sixth, this study did not examine impaired left ventricular diastolic performance, which is thought to occur early in diabetic cardiomyopathy. Finally, our study may not have enough power to test vendor differences because only eight studies reported other than EchoPAC software data.
Conclusion
Subclinical DCM can be detected by 2D-STE. Cardiac mechanics is impaired in all directions in patients with DM and exists in the LV, LA, and RV. The standardized reduction of strain was the largest in LA reservoir strain, closely followed by RVGLS and LVGLS. Higher BMI in adults with DM is associated with worse LV strain values, and higher HbA1c is associated with worse RVGLS.
Abbreviations
- DCM:
-
Diabetic cardiomyopathy
- LVGLS:
-
Left ventricular global longitudinal strain
- LVGCS:
-
Left ventricular global circumferential strain
- LVGRS:
-
Left ventricular global radial strain
- LVSR:
-
Left ventricular longitudinal systolic strain rate
- MD:
-
Mean difference
- RVGLS:
-
Right ventricular global longitudinal strain
- STE:
-
Speckle tracking echocardiography
- DM:
-
Diabetes mellitus
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107843
Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease: the framingham study. JAMA 241:2035–2038
Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J et al (2018) Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 20:853–872
From AM, Scott CG, Chen HH (2010) The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol 55:300–305
Di Bonito P, Moio N, Cavuto L, Covino G, Murena E, Scilla C et al (2005) Early detection of diabetic cardiomyopathy: usefulness of tissue Doppler imaging. Diabet Med 22:1720–1725
Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH (2003) Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol 41:611–617
Galderisi MJ (2006) Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol 48:1548–1551
Ernande L, Bergerot C, Rietzschel ER, De Buyzere ML, Thibault H, PignonBlanc PG et al (2011) Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr 24:1268–1275
Leung M, Wong VW, Hudson M, Leung DY (2016) Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ: Cardiovasc Imaging 9:e003643
Jørgensen PG, Jensen MT, Biering-Sørensen T, Mogelvang R, Fritz-Hansen T, Vilsbøll T et al (2018) Burden of uncontrolled metabolic risk factors and left ventricular structure and function in patients with type 2 diabetes mellitus. J Am Heart Assoc 7(19):e008856
Jensen MT, Sogaard P, Andersen HU, Bech J, Fritz Hansen T, Biering-Sorensen T et al (2015) Global longitudinal strain is not impaired in type 1 diabetes patients without albuminuria: the Thousand & 1 study. JACC Cardiovasc Imaging 8:400–410
Tadic M, Celic V, Cuspidi C, Ilic S, Pencic B, Radojkovic J et al (2015) Right heart mechanics in untreated normotensive patients with prediabetes and type 2 diabetes mellitus: a two- and three-dimensional echocardiographic study. J Am Soc Echocardiogr 28:317–327
Stevanovic A, Dekleva M (2018) Complications i. The importance of subclinical left ventricular dysfunction and blood pressure pattern in asymptomatic type-2 diabetic patients: the diagnostic and prognostic significance of Tissue Doppler parameters, left ventricular global longitudinal strain, and nighttime blood pressure during sleep. J Diabet Complicat 32:41–47
Bogdanović J, Ašanin M, Krljanac G, Lalić NM, Jotić A, Stanković S et al (2019) Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: an analysis based on two-dimensional speckle tracking echocardiography. Cardiovasc Diabetol 18:68
Haley JE, Zhiqian G, Philip KR, Nicolas ML, Thomas KR, Lawrence DM et al (2020) Reduction in myocardial strain is evident in adolescents and young adults with obesity and type 2 diabetes. Pediatr Diabetes 21:243–250
Suto M, Tanaka H, Mochizuki Y, Mukai J, Takada H, Soga F et al (2017) Impact of overweight on left ventricular function in type 2 diabetes mellitus. Cardiovasc Diabetol 16:145
Cameli M, Mandoli GE, Lisi E, Ibrahim A, Incampo E, Buccoliero G et al (2019) Left atrial, ventricular and atrio-ventricular strain in patients with subclinical heart dysfunction. Int J Cardiovasc Imaging 35:249–258
Roberts TJ, Barros-Murphy JF, Burns AT, MacIsaac RJ, MacIsaac AI, Prior DL et al (2020) Reduced exercise capacity in diabetes mellitus is not associated with impaired deformation or twist. J Am Soc Echocardiogr 29:29
Philouze C, Obert P, Nottin S, Benamor A, Barthez O, Aboukhoudir F (2018) Dobutamine stress echocardiography unmasks early left ventricular dysfunction in asymptomatic patients with uncomplicated Type 2 diabetes: a comprehensive two-dimensional speckle-tracking imaging study. J Am Soc Echocardiogr 31:587–597
Lin J-L, Sung K-T, Su C-H, Chou T-H, Lo C-I, Tsai J-P et al (2018) Cardiac structural remodeling, longitudinal systolic strain, and torsional mechanics in lean and nonlean dysglycemic Chinese adults. Circ: Cardiovas Imaging 11:e007047
Jørgensen PG, Jensen MT, Mogelvang R, Fritz-Hansen T, Galatius S, Biering-Sørensen T et al (2016) Impact of type 2 diabetes and duration of type 2 diabetes on cardiac structure and function. Int J Cardiol 221:114–121
Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC et al (2017) Association of insulin resistance and glycemic metabolic abnormalities with LV structure and function in middle age: the CARDIA study. JACC Cardiovasc Imaging 10:105–114
Loncarevic B, Trifunovic D, Soldatovic I, Vujisic-Tesic B (2016) Silent diabetic cardiomyopathy in everyday practice: a clinical and echocardiographic study. BMC Cardiovasc Disord 16:242
Jedrzejewska I, Krol W, Swiatowiec A, Wilczewska A, Grzywanowska-Laniewska I, Dluzniewski M et al (2016) Left and right ventricular systolic function impairment in type 1 diabetic young adults assessed by 2D speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging 17:438–446
Cohen J (2013) Statistical power analysis for the behavioral sciences, 2nd edn. Academic press, New York
Vo HQ, Marwick TH, Negishi K (2020) Pooled summary of native T1 value and extracellular volume with MOLLI variant sequences in normal subjects and patients with cardiovascular disease. Int J Cardiovasc Imaging 36:325–336
Voigt J-U, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R et al (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J—Cardiovas Imaging 16:1–11
Enomoto M, Ishizu T, Seo Y, Yamamoto M, Suzuki H, Shimano H et al (2015) Subendocardial systolic dysfunction in asymptomatic normotensive diabetic patients. Circ J 79:1749–1755
Zuo H, Yan J, Zeng H, Li W, Li P, Liu Z et al (2015) Diagnostic power of longitudinal strain at rest for the detection of obstructive coronary artery disease in patients with type 2 diabetes mellitus. Ultrasound Med Biol 41:89–98
Rasalingam R, Holland MR, Cooper DH, Novak E, Rich MW, Miller JG et al (2016) Patients with diabetes and significant epicardial coronary artery disease have increased systolic left ventricular apical rotation and rotation rate at rest. Echocardiography 33:537–545
Wierzbowska-Drabik K, Trzos E, Kurpesa M, Rechciński T, Miśkowiec D, Cieślik-Guerra U et al (2018) Diabetes as an independent predictor of left ventricular longitudinal strain reduction at rest and during dobutamine stress test in patients with significant coronary artery disease. Eur Heart J Cardiovasc Imaging 19:1276–1286
Fang ZY, Leano R, Marwick TH (2004) Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci 106:53–60
Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T (2008) The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. J Am Soc Echocardiogr 21:1138–1144
Cikes M (2016) Solomon SD Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 37:1642–1650
Shah AM, Solomon SD. (2012) Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Oxford University Press.
Ghoreyshi-Hefzabad SM, Jeyaprakash P, Gupta A, Vo HQ, Pathan F, Negishi K (2021) Three-dimensional global left ventricular myocardial strain reduced in all directions in subclinical diabetic cardiomyopathy: a systematic review and meta-analysis. J Am Heart Assoc 10:e020811
Ng AC, Prevedello F, Dolci G, Roos CJ, Djaberi R, Bertini M et al (2018) Impact of diabetes and increasing body mass index category on left ventricular systolic and diastolic function. J Am Soc Echocardiogr 31:916–925
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128
Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J et al (2016) Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabet Endocrinol 4:411–419
Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y (2009) Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr 10:926–932
Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M et al (2009) Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 104:1398–1401
Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M et al (2011) Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr 24:898–908
D’Andrea A, Nistri S, Castaldo F, Galderisi M, Mele D, Agricola E et al (2012) The relationship between early left ventricular myocardial alterations and reduced coronary flow reserve in non-insulin-dependent diabetic patients with microvascular angina. Int J Cardiol 154:250–255
Kadappu KK, Boyd A, Eshoo S, Haluska B, Yeo AE, Marwick TH et al (2012) Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J—Cardiovas Imaging 13:1016–1023
Conte L, Fabiani I, Barletta V, Bianchi C, Maria CA, Cucco C et al (2013) Early detection of left ventricular dysfunction in diabetes mellitus patients with normal ejection fraction, stratified by bmi: a preliminary speckle tracking echocardiography study. J Cardiovasc Ecogr 23:73–80
Tadic M, Ilic S, Cuspidi C, Ivanovic B, Bukarica L, Kostic N et al (2014) Left and right atrial phasic function and deformation in untreated patients with prediabetes and type 2 diabetes mellitus. Int J Cardiovasc Imaging 31:65–76
Zoroufian A, Razmi T, Taghavi-Shavazi M, Lotfi-Tokaldany M, Jalali A (2014) Evaluation of subclinical left ventricular dysfunction in diabetic patients: longitudinal strain velocities and left ventricular dyssynchrony by two-dimensional speckle tracking echocardiography study. Echocardiography 31:456–463
Bakirci EM, Demirtas L, Degirmenci H, Topcu S, Demirelli S, Hamur H et al (2015) Relationship of the total atrial conduction time to subclinical atherosclerosis, inflammation and echocardiographic parameters in patients with type 2 diabetes mellitus. Clinics 70:73–80
Karagoz A, Bezgin T, Kutluturk I, Kulahcioglu S, Tanboga IH, Guler A et al (2015) Subclinical left ventricular systolic dysfunction in diabetic patients and its association with retinopathy: a 2D speckle tracking echocardiography study. Herz 40(Suppl 3):240–246
Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J et al (2015) Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ: Heart Fail 8:448–454
Tadic M, Ilic S, Cuspidi C, Stojcevski B, Ivanovic B, Bukarica L et al (2015) Left Ventricular mechanics in untreated normotensive patients with Type 2 diabetes mellitus: A Two- and three-dimensional speckle tracking study. Echocardiography 32:947–955
Abdel-Salam Z, Khalifa M, Ayoub A, Hamdy A, Nammas W (2016) Early changes in longitudinal deformation indices in young asymptomatic patients with type 1 diabetes mellitus: assessment by speckle-tracking echocardiography. Minerva Cardioangiol 64:138–144
Bakhoum SWG, Habeeb HA, Elebrashy IN, Rizk MN (2016) Assessment of left ventricular function in young type 1 diabetes mellitus patients by two-dimensional speckle tracking echocardiography: Relation to duration and control of diabetes. Egypt Heart J 68:217–225
Mochizuki Y, Tanaka H, Matsumoto K, Sano H, Shimoura H, Ooka J et al (2016) Impaired mechanics of left ventriculo-atrial coupling in patients with diabetic nephropathy. Circ J 80:1957–1964
Tadic M, Cuspidi C, Vukomanovic V, Ilic S, Celic V, Obert P et al (2016) The influence of type 2 diabetes and arterial hypertension on right ventricular layer-specific mechanics. Acta Diabetol 53:791–797
Tadic M, Vukomanovic V, Cuspidi C, Suzic-Lazic J, Stanisavljevic D, Celic V (2017) Left atrial phasic function and heart rate variability in asymptomatic diabetic patients. Acta Diabetol 54:301–308
Tadic M, Vukomanovic V, Cuspidi C, Suzic-Lazic J, Pencic-Popovic B, Radojkovic J et al (2017) The relationship between right ventricular deformation and heart rate variability in asymptomatic diabetic patients. J Diabet Complicat 31:1152–1157
Vukomanovic V, Tadic M, Suzic-Lazic J, Kocijancic V, Celic V (2017) The relationship between heart rate variability and left ventricular layer-specific deformation in uncomplicated diabetic patients. Int J Cardiovasc Imaging 33:481–490
Ahmed TA, Ahmed YA, Arafa AI, Salah RA (2018) Detection of occult right ventricular dysfunction in young Egyptians with type 1 diabetes mellitus by two-dimensional speckle tracking echocardiography. Indian Heart J 70:665–671
Ringle A, Dornhorst A, Rehman MB, Ruisanchez C, Nihoyannopoulos P (2017) Evolution of subclinical myocardial dysfunction detected by two-dimensional and three-dimensional speckle tracking in asymptomatic type 1 diabetic patients: a long-term follow-up study. Echo Res 4:73–81
Tadic M, Cuspidi C, Vukomanovic V, Ilic S, Obert P, Kocijancic V et al (2018) Layer-specific deformation of the left ventricle in uncomplicated patients with type 2 diabetes and arterial hypertension. Arch Cardiovasc Dis 111:17–24
Berceanu M, Mirea O, Târtea G-C, Donoiu I, Militaru C, Istrătoaie O et al (2019) The significance of right ventricle in young subjects with diabetes mellitus type 1. An Echocardiographyic study. Curr Health Sci J 45:174
Acknowledgements
The authors thank Mr. Lajos Bordas, for his support in systematic review.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Dr. Negishi is supported by a Fellowship (Award Reference No.101868) from the National Heart Foundation of Australia.
Author information
Authors and Affiliations
Contributions
SM.GH. and A.P. collected the data through systematic review. SM.GH., P.J, and H.V performed statistical analyses. SM.GH., P.J. and A.P. wrote the initial draft. F.P and K.O provided essential edit of the manuscript. K.N. conceived, designed, and supervised the analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The Author(s) declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghoreyshi-Hefzabad, SM., Jeyaprakash, P., Vo, H.Q. et al. Subclinical systolic dysfunction detected by 2D speckle tracking echocardiography in adults with diabetes mellitus: systematic review and meta-analysis of 6668 individuals with diabetes mellitus and 7218 controls. Int J Cardiovasc Imaging 39, 977–989 (2023). https://doi.org/10.1007/s10554-023-02810-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02810-4