Abstract
Background
Hyperglycemia has detrimental effect on ischemic myocardium, but the impact of acute hyperglycemia on the myocardium in asymptomatic diabetic patients has not been fully elucidated. Thus, this follow-up study was aimed to investigate the effects and reversibility of acute hyperglycemia on regional contractile function of left ventricle (LV) in diabetic patients without cardiovascular disease.
Methods
The two-dimensional speckle tracking echocardiography (2D-STE), including multilayer strain analysis, was used for evaluation of global and regional LV function in asymptomatic, normotensive patients with uncomplicated diabetes, with acute hyperglycemia ( ≥ 11.1 mmol/l) (Group A, n = 67), or with optimal metabolic control (fasting plasma glucose < 7 mmol/l and HbA1c < 7%) (Group B, n = 20), while 20 healthy individuals served as controls (Group C). In group A, after 72 h of i.v. continuous insulin treatment (at the time euglycemia was achieved) (second examination) and after 3 months following acute hyperglycemia (third examination) 2D-STE was repeated.
Results
Global longitudinal strain (GLS) (− 19.6 ± 0.4%) in Group A was significantly lower in comparison to both groups B (− 21.3 ± 0.4%; p < 0.05) and C (− 21.9 ± 0.4%; p < 0.01) at baseline, while we could not detect the differences between groups B and C. Peak systolic longitudinal endocardial (Endo), mid-myocardial (Mid) and epicardial (Epi) layer strain were significantly lower in group A at baseline compared to both groups B and C. Deterioration in peak systolic circumferential strain was observed at basal LV level, in all three layers (Endo, Mid and Epi) and in mid-cavity LV level in Epi layer in group A in comparison to group C. Moreover, in group A, after euglycemia was achieved (at second and third examination) GLS, as well as peak longitudinal and circumferential strain remain the same.
Conclusion
Acute hyperglycemia in asymptomatic diabetic patients has significant negative effects on systolic LV myocardial mechanics primarily by reducing GLS and multilayer peak systolic longitudinal and circumferential strain which was not reversible after three months of good glycemic control.
Similar content being viewed by others
Background
Diabetes, as a chronic metabolic disease, is characterized by elevated blood glucose levels (hyperglycemia) that can lead to specific vascular complications, including heart failure, while cardiovascular complications are the leading cause of morbidity and mortality in these patients [1, 2]. A sudden increase of blood glucose levels (acute hyperglycemia) has been documented to occur in people with previously diagnosed diabetes or impaired glucose tolerance. Acute hyperglycemia is usually defined according to Ishihara [3] as blood glucose levels which are at hospital admission measured to be ≥ 11.1 mmol/l. Over the years, numerous studies have shown that acute hyperglycemia has many harmful effects on the cardiovascular system. It causes insufficiency, dysregulation and overall endothelial dysfunction [4] and reduces: collateral circulation [5], spontaneous reperfusion in patients with acute myocardial infarction (AMI) with ST-segment elevation [6] and it deepens the QT [7]. In addition, some studies have shown that, during AMI, acute hyperglycemia is associated with impaired function (dysfunction) of the left ventricle (LV) [8, 9].
Recently, a new two-dimensional speckle tracking echocardiography (2D-STE) has been developed which enables reliable assessment of regional deformation in three directions: longitudinal, circumferential and radial. With the use of this technique it is possible to measure myocardial function parameters and quantify global and regional systolic and diastolic myocardial function [10]. Also, this technique allows to track and display myocardial deformation through its’ different layers [11]. In that context, it has been previously shown that the magnitude of LV longitudinal strain at the basal level decreases, whereas the apical LV longitudinal strain increases with increasing age, while presence of diabetes modulate the effect of age on LV strain [12]. Similarly, hypertension and obesity, both in asymptomatic diabetic and nondiabetic patients induce sequential impairment of LV function from the endocardium to the epicardium [13, 14]. Finally, in asymptomatic, normotensive diabetic patients (both type 1 and type 2) with normal LVEF significant subclinical LV systolic dysfunction was found [15, 16]. Therefore, layer-specific evaluation of LV strain could provide a better understanding of the effect of acute hyperglycemia on LV systolic function in asymptomatic diabetic patients.
Most previous research is focused on assessing impacts that acute hyperglycemia has on the myocardium during AMI and there is no study concerning it’s effects on the contractile function and any early abnormalities of the heart, determined using 2D-STE, in diabetic patients without previous cardiovascular disease (CVD). Thus, the aim of this study was to investigate the effects of acute hyperglycemia on regional function of the LV, as well as to determine the reversibility of possible myocardial changes following euglycemia in diabetic patients who do not present any symptoms and clinical signs of CVD.
Material and methods
Study population
A total of 67 patients with diabetes (Group A), 30 with type 1 diabetes (T1D) and 37 with type 2 diabetes (T2D), both gender (45 males), with an average age of 38.3 ± 1.6 years that had acute hyperglycemia (plasma glucose ≥ 11.1 mmol/l) were included in this prospective study which was performed over a period of 4 years (from January 2014 to January 2018). Patients included in this study were carefully selected from the patients admitted to Intensive Care Unit (ICU) according to the following inclusion criteria: (1) previously known diabetes (32 patients, 47.8%) or newly onset diabetes (35 patients, 52.2%) with hyperglycemia (plasma glucose ≥ 11.1 mmol/l) on the admission; (2) free of previously known or newly diagnosed CVD (including hypertension) or on treatment for CVD or hypertension; (3) free of known or newly diagnosed microvascular diabetic complications. Those patients younger than 18 or older than 65 years of age, with unstable angina pectoris, AMI or history of coronary interventions, heart failure, atrial fibrillation or irregular rhythm on ECG, malignant diseases, obstructive pulmonary disease, hepatic or renal failure (eGFR ≤ 60 ml/min/1.73 m2), acute or chronic infections, ketoacidosis, treated with corticosteroids or immunosuppressive agents and with new uncontrolled hypertension (blood pressure higher than 180/110 mmHg) requiring treatment were excluded from the study.
For comparison, there were two control groups: (1) Group B (n = 20, 10 males, 43.2 ± 3.6 years old) consisting of carefully selected patients with diabetes who had optimal metabolic control (HbA1c ≤ 7.0%) with fasting plasma glucose < 7.0 mmol/l at the time of inclusion in the study and without any known or newly diagnosed micro- or macrovascular diabetic complications; (2) Group C consisting of healthy volunteers (n = 20, 8 males, 36.2 ± 2.0 years old).
This study has been approved by the Institutional Ethics Committee and all patients have signed the informed consent form prior to inclusion. All of the investigated patients (Group A) were admitted to the ICU of the Clinic for Endocrinology, Diabetes and Metabolic Diseases, Clinical Center of Serbia, and main reason for admission was hyperglycemia. The echocardiographic examination was exerted by experienced cardiologist in the Clinic for Cardiology, Clinical Center of Serbia, while all biological samples were processed at Center for Medical Biochemistry, Clinical Center of Serbia.
Research design
Upon the admission to ICU in all investigated patients (Group A) a full medical history was obtained and physical exam with anthropometric measurements were undertook. Body mass index (BMI) was calculated according to the standard formula (kg/m2) from body weight and height measured with a digital scale. Intravenous cannulas were inserted in antecubital vein of both arms for blood sampling, as well as for saline, glucose and insulin infusion for the next 72 h. First, blood was drowning for basal laboratory analysis and immediately after that all patients underwent a resting standard echocardiographic examination before any antihyperglycemic therapy was started.
All investigated patients were subjected to the same therapeutic protocol until the point when euglycemia (plasma glucose ≤ 6.0 mmol/l) was achieved (by the 72 h). Patients were treated with a solution of rapid acting human insulin (Actrapid, Novo Nordisk, Denmark) dissolved in a physiological saline solution (Hemofarm, Vršac, Serbia) (1 IU of insulin in 1 ml of NaCl 0.9% saline solution) and apply by using continuous insulin infusion pump (Perfusor Space Infusion, BBraun, Germany) starting at a rate of 6 IU/h. Glycemic values were taken every hour (AccuChek glucometer, Roche, Germany) and infusion rate was further adjusted according to the glucose levels using the standard protocol in order to achieve near-normal glycemia. Additional saline salts, 5% glucose solution, electrolyte replacement and antibiotics were also implemented i.v. At the time euglycemia was achieved (after 72 h of i.v. continuous insulin treatment) and after three months ( ± 15 days) following acute hyperglycemia biochemical analyses and echocardiography examinations (done by the same cardiologist) were repeated.
Subjects in control groups (Groups B and C) were examined only once when a medical history data was obtained together with anthropometric parameters, laboratory tests and an echocardiogram was performed.
Echocardiographic examination
All resting standard echocardiographic examinations were performed using Vivid E9 (General Electric). Data were acquired with a 3.5 MHz transducer in the parasternal (long- and short-axis views) and apical views (two- and four-chamber and apical long-axis views). Echocardiographic methods as M-mode, 2D, color Doppler, pulse Doppler and continue Doppler were performed. All definitions and rule for measurements were in accordance with the recommendations of the European and American Society of Echocardiography [17]. The LV end-diastolic (EDV) and end-systolic volumes (ESV) were measured from the apical two- and four-chamber views, and LV ejection fraction (EF) was calculated using the Simpson’s rule [17]. LV diastolic function was evaluated using early (E-wave) and late (A-wave) transmitral velocities, the E/A ratio and the E-deceleration time (DT) obtained by the spectral pulsed-wave Doppler recordings and by tissue Doppler imaging. The peak early diastolic velocity (E′) was measured at the basal myocardial segments, on the apical four-chamber view, at mitral annulus level (Em′ for the septal and El′ for the lateral) and average value was considered. Finally E/E′ ratio was calculated [17].
2D left ventricular speckle-tracking strain analyses
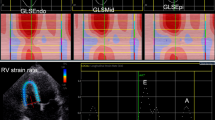
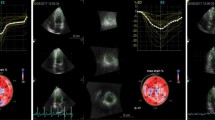
The two-dimensional speckle tracking echocardiography (2D-STE), as noninvasive ultrasound imaging technique was used for an objective and quantitative evaluation of global and regional myocardial function. The recordings with a frame rate between 50 and 70 frames/s were performed and analysed offline using General Electric software (EchoPAC software version 113 GE Medical Systems). Standard 2D gray-scale images of the LV were acquired at apical four-, two-chambers and apical long-axis view (Fig. 1), as well as parasternal short-axis view at base (mitral valve), papillary muscles and axis level. Global longitudinal LV myocardial strain (GLS) and systolic longitudinal, circumferential and radial strain were calculated offline. Longitudinal, circumferential and radial strain is analyzed on the 18-segment segmentation model. However, radial strain was not further processed because of a problem using 2D acquisition methods or a low number of high-quality images and suboptimal tracking which influenced the consistency of findings. Multilayer longitudinal and circumferential strains were determined by 2D-STE software which automatically creates a region of interest, which contained subendocardial (Endo), mid-myocardial (Mid) and subepicardial (Epi) layers (Fig. 2). Multilayer longitudinal strain was assessed in apical four-chamber, two-chamber and long-axis views, whereas multilayer circumferential strain was evaluated in the short-axis at the three levels as mentioned [18]. In randomly selected 15 patients, 2–4 weeks after performing the initial measurements, strain analysis was repeated by the same observer (G.K.) to determine intra-observer reproducibility. The absolute difference between two measurements divided by the average value of these two measurements was calculated together with interclass correlation coefficient (ICC).
An example of a color-coded two-dimensional speckle tracking echocardiography (2D-STE) of the left ventricle (LV) and corresponding time–strain curves from 18 LV segments derived from the apical 4-, 2-chamber and long-axis views for measurement of global longitudinal strain (GLS) in diabetic patient with acute hyperglycemia. GLS was calculated as the average peak strain of 18 LV segments, and in this example the value of GLS is − 22.4%
Multilayer longitudinal and circumferential strains determined by two-dimensional speckle tracking echocardiography (2D-STE) in diabetic patient with acute hyperglycemia. a Subendocardial (Endo), mid-myocardial(Mid) and subepicardial(Epi) layers of longitudinal strain assessed in apical long-axis views; b Endo, Mid and Epi layers of circumferential strain at base (mitral valve) evaluated in parasternal short-axis view
Laboratory analyses
Plasma glucose concentration (commercial test reagent, Abbott Diagnostic) and HbA1c levels (commercial test reagent, SEBIA, France) were determined by spectrophotometry. Serum lipid levels (total and HDL cholesterol (Ch) and triglycerides) were analyzed enzymatically using commercial kit (ADVIA Chemistry), while LDL-Ch was calculated by standard Friedewald formula. Serum concentrations of troponin (commercial test reagent Roche Diagnostics) and N terminal brain fragment (B-type) for natriuretic peptide (NT-proBNP; commercial test reagent Abbott Diagnostics) were determined by the CLIA test.
Statistical analysis
All continuous variables are presented as mean ± SE, while categorical data are presented as percentages. Sample size for the study was calculated according to previously published results using standard methods with probability (power) 0.8 and type I error probability 0.05 and we estimated that minimum number of investigated patients (Group A) should be 61. The differences between the groups were tested using a one-way analysis of variance (ANOVA) with Bonferroni post hoc test, while Kruskal–Wallis test was used for categorical variables. The statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc., USA). The level of statistical significance was set at p < 0.05.
Results
Characteristics of the study populations
Baseline demographic and anthropometric characteristics of the investigated subjects are presented in Table 1. All three groups did not differ in respect to gender, age, body weight and BMI. Proportion of patient with T1D and T2D was similar in group A and group B, as well as average duration of diabetes. The analysis of the previous medication revealed similar pattern the drugs used related to diabetes (oral hypoglycemic agents or insulin), while none of the investigated subjects used any cardiovascular drugs.
Laboratory measurements
The biochemistry analyses performed in the investigated patient groups are shown in Table 2. At baseline, we found significantly higher blood glucose levels in group A (upon hospital admission; first examination) than in both groups B and C (p < 0.001, respectively), while there were no differences when we compared groups B and C. After 72 h hours of insulin treatment in group A (second examination) we achieved normalization of the glycemic values which slightly increase after three months (third examination) (p < 0.001). Similarly, HbA1c level were significantly higher in group A in comparison to both groups B and C (p < 0.001, respectively) and significantly decreased after three months in group A (p < 0.001).
Regarding the lipid parameters, total and LDL-Ch at baseline were similar between the groups, although we found in group A significantly lower levels of both total and LDL-Ch levels at second and third examinations (p < 0.05). In contrast, HDL-Ch was significantly lower at baseline in group A vs both groups B and C (p < 0.001, respectively) and remain unchanged at second and third examinations in group A, while we could not find any differences between groups B and C.
Also, triglycerides value was significantly higher at baseline when we compared group A with both groups B and C (p < 0.05, respectively). In group A, triglycerides significantly decreased at second examination (p < 0.05) and remain unchanged at third examination (after three months).
Simultaneously, the levels of troponin and NT-proBNP were similar across all three groups at baseline, while we registered significant lowering of both parameters at second and third examination in group A (p < 0.05 and p < 0.001, respectively).
Conventional echocardiography
LV EDV and ESV, LV EF, as well as left atrium volume index (LAVI) were similar between the groups at baseline. Also, there were no changes in all those parameters in group A at second and third examinations (Table 3).
When we analyzed LV diastolic function we found that both diabetes groups A and B have significantly lower transmitral E wave and E/A ratio compared to healthy controls (group C), while we did not find any differences in transmitral A wave. E/E’ ratio had a trend to be higher in group A compared to both groups B and C at baseline, but did not reach statistical significance. Additionally, in group A, observed lower E and E/A ratio remained unchanged after euglycemia was achieved (second examination) or after 3 months (third examination) (Table 3). In contrast, although there were no significant differences in DT across the groups at baseline, we found that in group A, DT was significantly shorter at both second and third examinations in comparison to baseline (p < 0.001) (Table 3).
2D LV speckle-tracking strain analyses
We found significantly lower GLS (− 19.6 ± 0.4%) in Group A in comparison to both groups B (− 21.3 ± 0.4%; p < 0.05) and C (− 21.9 ± 0.4%; p < 0.01) at baseline, while we could not detect the differences between groups B and C. Moreover, in group A, after euglycemia was achieved (second examination) we did not find any changes in GLS (− 19.0 ± 0.3%; p = ns) which remain the same at third examination (after 3 months) (− 19.6 ± 0.3%; p = ns) (Fig. 3).
When we analyzed peak systolic longitudinal strain, we detected that Endo, Mid and Epi myocardial layers strain are significantly lower in group A at baseline compared to both groups B and C (Table 4). In addition, observed lower Endo, Mid and Epi peak systolic longitudinal strain in group A remain unchanged at second and third examinations (Table 5).
Similarly, we also found deterioration in peak systolic circumferential strain: at basal LV level, all three layers strain (Endo, Mid and Epi) were significantly lower in group A in comparison to group C, while only Mid layer strain was lower in comparison to both groups B and C (Table 4). However, identified changes in group A have not improved at second (after euglycemia achieved) or third examination (after 3 months) (Table 5). In contrast, at mid-cavity LV level, only in Epi layer we found significantly lower peak systolic circumferential strain in group A vs group C, but not vs group B, which remain unchanged during the follow up period (Tables 4, 5). In apical LV level, there were no difference in peak systolic circumferential strain at any layer (Endo, Mid and Epi) among the investigated groups (Table 4).
The intra-observer variability for longitudinal and circumferential strain was 4.2% and 5.1% (respectively). ICC for intra-observer variability of the corresponding values was 0.899 (95% CI 0.607–0.969; p < 0.001) and 0.721 (95% CI 0.220–0.904; p < 0.01).
Discussion
Results from this study indicate that acute hyperglycemia (plasma glucose ≥ 11.1 mmol/l) in diabetic patients without any CVD and with preserved LVEF have significant negative effects on systolic LV function primarily by reducing GLS, peak systolic longitudinal (at Endo, Mid and Epi layers) and circumferential strain (at all three layers in basal level). Moreover, observed changes in systolic LV functions did not improve short-term after euglycemia was achieved (after 72 h) and remain unchanged even after longer period (three months) of good glycemic control.
LV diastolic dysfunction in diabetic patients
Previous investigations have been suggested that in patients with diabetes cardiac dysfunction (such as LV diastolic dysfunction, but also LV systolic dysfunction), defined as separate entity “diabetic cardiomyopathy”, could be detected very early in the clinical course of diabetes, independent of other cardiac risk factors (coronary artery diseases or hypertension) which could progress to heart failure [19, 20]. In that context, echocardiographic studies confirm early diastolic dysfunction in asymptomatic T2D patients [21] which correlate with the level of HbA1c and that a 1% increase in HbA1c was associated with 8% increase of the heart failure risk, independent of other CV risk factors [22]. On the other hands, studies in children and adolescents with T1D found diastolic dysfunction despite intensive insulin treatment and good metabolic control [23,24,25] demonstrating the existence of high risk for cardiac dysfunction in those patients, independent of the HbA1c level. Similarly, 4 month of strict glucose control in T2D patients, insulin based, does not improve the parameters of diastolic dysfunction in T2D [26]. Moreover, important echocardiographic changes in diastolic function were observed even in patients with prediabetes [27] suggesting that metabolic milieu in T2D, not only chronic hyperglycemia, could influence the cardiac diastolic function. We found in our study that both groups of diabetic patients, with poor and good metabolic control (both groups A and B), have similar changes in E velocity and E/A ratio implying the presence of diastolic dysfunction (regardless of HbA1c level) in comparison to healthy controls. Interestingly, according to our results, acute hyperglycemia does not additionally impair observed LV diastolic dysfunction in these groups of diabetic patients, except the identified changes in transmitral DT which is slightly, but not significantly increase compared to controls. After euglycemia was achieved, DT significantly decline implying that changes in LV diastolic filling patterns might be one of the consequences of acute hyperglycemia in diabetes.
Layer-specific LV systolic dysfunction in diabetic patients
Strain analysis derived from 2D-STE enables detection of subclinical myocardial systolic dysfunction beyond conventional LV echocardiographic assessment in all three myocardial layers. Studies on layer-specific LV multidirectional strain reported impairments in both longitudinal and circumferential strains in asymptomatic T2D patients which correlated with diabetes duration [28] or overweight [29, 30], while others found longitudinal systolic dysfunction and impairment associated with subendocardial wall thickening, but in poorly controlled asymptomatic and normotensive T2D patients [31]. In addition, STE-derived layer-specific cardiac dysfunctions correlate with the markers of histological remodeling (hypertrophy and fibrosis) and with progression of untreated diabetes in animal models [32, 33]. Using more accurate and sensitive technique, contrast-enhanced cardiovascular magnetic resonance, layer-specific subclinical systolic dysfunction was detected in uncomplicated T2D patients which was associated with impaired coronary microvascular perfusion [34]. Also, it was shown in asymptomatic T2D patients with good glycemic control presence of impairment of LV longitudinal strain but preserved circumferential and radial strain [35], as well as changes in GLS which could be important prognostic factor for the future CV event [36, 37]. In contrast, in 390 patients with chronic ischemic cardiomyopathy (25% with diabetes) endocardial circumferential strain detected by 2D-STE appears to be a better predictor of future cardiac events than total myocardial scar assessed by cardiac magnetic resonance imaging [38]. Very recently, detail analysis of LV mechanics in asymptomatic, hypertensive diabetic patients revealed lower longitudinal strain in Endo layer and lower circumferential strain in Endo and Mid layer compared to healthy controls, which also correlated significantly with HbA1c levels [39]. However, in the study of T2D patients, stratified according to the overall metabolic risk factors, patients with well-control risk factors (including near-normal level of HbA1c), similar to our group B, had only LV diastolic impairment, but there was no evidence of structural or systolic changes of the LV function detected by strain analysis [40].
Effect of acute hyperglycemia on layer-specific LV strain in diabetic patients
In our group of asymptomatic, normotensive diabetic patients with strict metabolic control (mean HbA1c 6.1 ± 1.0%; Group B) we did not find any statistically significant differences in GLS, peak longitudinal or circumferential strain in comparison to healthy controls (Table 4, Fig. 3). In contrast, in diabetic patients with really poor metabolic control and acute hyperglycemia (Group A, mean HbA1c 11.5 ± 0.3% and glycaemia 22.5 ± 1.1 mmol/l) we found significantly lower GLS, peak longitudinal strain in Endo, Mid and Epi layer, peak circumferential strain in all three layers at basal LV level and in Epi layer in mid-cavity LV level, compared to healthy controls and diabetic patients with good glycemic control (Table 4, Fig. 3). Thus, these results suggest that acute hyperglycemia in asymptomatic diabetic patients have negative impact dominantly on LV systolic function at all three layers (longitudinal and circumferential strain).
To the best of our knowledge, this is the first study investigating the effect of acute hyperglycemia on LV diastolic and systolic function using 2D-STE in diabetic patients without CVD or hypertension. Our results shown that acute hyperglycemia in asymptomatic diabetic patients induces significant LV systolic changes in multilayer myocardial strain, particularly in GLS, peak longitudinal and circumferential strain which remain unchanged after short-term (after 72 h) and long-term (after 3 months) euglycemia was achieved.
Assessment of the effects of acute hyperglycemia on the LV contractile function in diabetic patients has only been previously the subject of a few research papers. In one study, Nielsen R et al. [41] unexpectedly revealed that short-term hyperglycemia (9–12 h) induced by insulin discontinuation, increased LV contractile function (detected as strain rate) in relatively small number (20) of T2D patients with or without heart failure. This result was contrary to previously findings that hyperglycemia is considered harmful in patients with heart failure, particularly when heart failure coexists with diabetes [42]. However, in this mentioned study [41], after insulin discontinuation the level of blood glucose rise till mean 9.9 ± 2.1 mmol/l in diabetic patients without heart failure, while in our group of asymptomatic diabetic patients hyperglycemia was significantly higher and we analyzed not only global strain (like in Nielsen study) [41], but all three LV myocardial layers which could explain different results.
Some of the previously conducted studies have shown that hyperglycemia following a glucose infusion in diabetic patients with normal LVEF, had no effect on the global LV function [43] detected by standard echocardiography, while we used more sophisticated method (strain analysis) for detection of LV systolic function. Studies in animal models indicated that acute hyperglycemia could suppress LV diastolic function and influences mitochondrial energy signaling [44], or induces changes in polyol metabolic pathway with consequently increase in oxidative stress leading to myocardial contractile dysfunction [45]. Compelling evidence is accumulating which suggests a role of oxidative stress as a pathogenic factor underlying the negative effect of acute hyperglycemia on cardiovascular system [9] which could partly explain our results.
Finally, according to our result, the observed changes in LV systolic function during acute hyperglycemia are not reversible neither after short-term (72 h) nor after longer (3 months) period of good glycemic control, although some studies have shown that amelioration in glycemic control over a 1 year period led to improvements in LV systolic function [46]. Several studies suggest the concept that negative early effect of hyperglycemia on cells in diabetes is remembered, the phenomenon named “metabolic memory”, primarily based on mitochondrial damages, overproduction of reactive oxygen species and oxidative stress [47]. Considering that phenomenon, it could be possible that the risk of hyperglycemia-related vascular complications in diabetes persist even when hyperglycemia is normalized, as previously shown in long-term (several years) trials [48, 49]. However, in our study, the follow-up period with good glycemic control was only three months which possibly would not be long enough to see improvement in the observed LV systolic dysfunction.
Research limitations
The present study has several possible limitations. First, our study population is relatively small and consists of both T1D and T2D patients, but the separate analysis has shown the same results, regardless of diabetes type. Thus, results are presented for whole diabetes patients group and control diabetes group (Group B) was adjusted for type of diabetes. Second, we could not exclude subclinical coronary artery disease in our diabetes patients because stress test was not performed. Third, due to the GE EchoPAC version 113 software limitations, the images are two-dimensional, and once the ‘dots’ on the image ‘move out’ of the plane during a cardiac cycle they cannot be monitored by this software.
Conclusion
The acute hyperglycemia in asymptomatic diabetic patients with preserved LVEF have significant negative effects on systolic LV myocardial mechanics primarily by reducing GLS, peak systolic longitudinal (at Endo, Mid and Epi layers) and peak circumferential strain (at all three layers in basal LV level). Moreover, observed changes in systolic LV functions was not reversible after three months of good glycemic control.
Availability of data and materials
The datasets generated and analyzed for this study are available from the corresponding author upon reasonable request.
Abbreviations
- 2D-STE:
-
two-dimensional speckle tracking echocardiography
- AMI:
-
acute myocardial infarction
- BMI:
-
body mass index
- Ch:
-
cholesterol
- CVD:
-
cardiovascular disease
- DT:
-
deceleration time
- EDV:
-
end-diastolic volume
- EF:
-
ejection fraction
- Endo:
-
subendocardial layer
- Epi:
-
subepicardial layer
- ESV:
-
end-systolic volumes
- GLS:
-
global longitudinal strain
- ICC:
-
interclass correlation coefficient
- ICU:
-
Intensive Care Unit
- LAVI:
-
left atrium volume index
- LV:
-
left ventricle
- Mid:
-
mid-myocardial layer
- NT-proBNP:
-
N terminal brain fragment (B-type) for natriuretic peptide
- T1D:
-
type 1 diabetes
- T2D:
-
type 2 diabetes
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–502.
Ishihara M. Acute Hyperglycemia in Patients with Acute Myocardial Infarction. Circ J. 2012;76:563–71.
Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest. 2001;108(4):635–6.
Kersten JR, Toller WG, Tessmer JP, Pagel PS, Warltier DC. Hyperglycemia reduces coronary collateral blood flow through a nitric oxide-mediated mechanism. Am J Physiol Heart Circ Physiol. 2001;281(5):H2097–H3104.
Timmer JR, Ottervanger JP, de Boer MJ, Dambrink JH, Hoorntje JC, Gosselink AT, Suryapranata H, Zijlstra F, van 't Hof AW, Myocardial Infarction Study Group. Hyperglycemia is an important predictor of impaired coronary flow before reperfusion therapy in ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2005;45(7):999–1002.
Fiorentini A, Perciaccante A, Valente R, Paris A, Serra P, Tubani L. The correlation among QTc interval, hyperglycaemia and the impaired autonomic activity. Auton Neurosci. 2010;154(1–2):94–8.
Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Umemura T, Nakamura S, Yoshida M. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am Heart J. 2003;146(4):674–8.
Ceriello A. Acute hyperglycaemia: a ‘new’ risk factor during myocardial infarction. Eur Heart J. 2005;26:328–31.
Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zacà V, Ballo P, D'Andrea A, Muraru D, Losi M, Agricola E, D'Errico A, Buralli S, Sciomer S, Nistri S, Badano L, Echocardiography Study Group Of The Italian Society Of Cardiology (Rome, Italy). Speckle-tracking echocardiography A new technique for assessing myocardial function. J Ultrasound Med. 2011;30(1):71–83.
Adamu U, Schmitz F, Becker M, Kelm M, Hoffmann R. Advanced speckle tracking echocardiography allowing a three-myocardial layer-specific analysis of deformation parameters. Eur J Echocardiogr. 2009;10(2):303–8.
Abou R, Leung M, Khidir MJH, Wolterbeek R, Schalij MJ, AjmoneMarsan N, Bax JJ, Delgado V. Influence of aging on level and layer-specific left ventricular longitudinal strain in subjects without structural heart disease. Am J Cardiol. 2017;120(11):2065–72.
Kim SA, Park SM, Kim MN, Shim WJ. Assessment of left ventricular function by layer-specific strain and its relationship to structural remodeling in patients with hypertension. Can J Cardiol. 2016;32(2):211–6.
Blomstrand P, Sjöblom P, Nilsson M, Wijkman M, Engvall M, Länne T, Nyström FH, Östgren CJ, Engvall J. Overweight and obesity impair left ventricular systolic function as measured by left ventricular ejection fraction and global longitudinal strain. Cardiovasc Diabetol. 2018;17(1):113.
Jedrzejewska I, Krol W, Swiatowiec A, Wilczewska A, Grzywanowska-Laniewska I, Dluzniewski M, Braksator W. Left and right ventricular systolic function impairment in type 1 diabetic young adults assessed by 2D speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17(4):438–46.
Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr. 2009;10:926–32.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–71.
Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16(1):1–11.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycemia and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–8.
vonBibra H, Sutton MSJ. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia. 2010;53:1033–45.
Patil VC, Patil HV, Shah KB, Vasani JD, Shetty P. Diastolic dysfunction inasymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res. 2011;2:213–22.
Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, Selby JV. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–733.
Brunvand L, Fugelseth D, Stensaeth KH, Dahl-Jorgensen K, Margeirsdottir HD. Early reduced myocardial diastolic function in children and adolescents with type 1 diabetes mellitus apopulation-based study. BMC Cardiovasc Disord. 2016;16:103.
Suran D, Sinkovic A, Naji F. Tissue Doppler imaging is a sensitive echocardiographic technique to detect subclinical systolic and diastolic dysfunction of both ventricles in type 1 diabetes mellitus. BMC Cardiovasc Disord. 2016;16:72.
Yoldas T, Orun UA, Sagsak E, Aycan Z, Kaya O, Ozgur S, Karademir S. Subclinical left ventricular systolic and diastolic dysfunction in type 1 diabetic children and adolescents with good metabolic control. Echocardiography. 2018;35(2):227–33.
Jarnert C, Landstedt-Hallin L, Malmberg K, Melcher A, Ohrvik J, Persson H, Rydén L. A randomized trial of the impact of strict glycaemic control on myocardial diastolic function and perfusion reserve: a report from the DADD (Diabetes mellitus And Diastolic Dysfunction) study. Eur J Heart Fail. 2009;11(1):39–47.
Di Pino A, Mangiafico S, Urbano F, Scicali R, Scandura S, D'Agate V, Piro S, Tamburino C, Purrello F, Rabuazzo AM. HbA1c Identifies subjects with prediabetes and subclinical left ventricular diastolic dysfunction. J Clin Endocrinol Metab. 2017;102(10):3756–64.
Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr. 2009;10(8):926–32.
Suto M, Tanaka H, Mochizuki Y, Mukai J, Takada H, Soga F, Dokuni K, Hatani Y, Hatazawa K, Matsuzoe H, Sano H, Shimoura H, Ooka J, Matsumoto K, Hirota Y, Ogawa W, Hirata KI. Impact of overweight on left ventricular function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):145.
Musaeus KD, Pareek M. Body mass index, type 2 diabetes, and left ventricular function. Cardiovasc Diabetol. 2018;17(1):3.
Enomoto M, Ishizu T, Seo Y, Yamamoto M, Suzuki H, Shimano H, Kawakami Y, Aonuma K. Subendocardial systolic dysfunction in asymptomatic normotensive diabetic patients. Circ J. 2015;79(8):1749–55.
Mátyás C, Kovács A, Németh BT, Oláh A, Braun S, Tokodi M, Barta BA, Benke K, Ruppert M, Lakatos BK, Merkely B, Radovits T. Comparison of speckle‑tracking echocardiography with invasive hemodynamics for the detection of characteristic cardiac dysfunction in type‑1 and type‑2 diabetic rat models. Cardiovasc Diabetol. 2018;17(1):13.
Qiao YY, Zeng M, Li RJ, Leng ZT, Yang J, Yang Y. Layer-specific myocardial strain analysis: investigation of regional deformation in a rabbit model of diabetes mellitus during different stages. Med Ultrasonogr. 2016;18(3):339–44.
Liu X, Yang ZG, Gao Y, Xie LJ, Jiang L, Hu BY, Diao KY, Shi K, Xu HY, Shen MT, Ren Y, Guo YK. Left ventricular subclinical myocardial dysfunction in uncomplicated type 2 diabetes mellitus is associated with impaired myocardial perfusion: a contrast-enhanced cardiovascular magnetic resonance study. Cardiovasc Diabetol. 2018;17(1):139.
Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–401.
Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, Fang ZY, Prins JB, Stanton T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101:1061–6.
Liu JH, Chen Y, Yuen M, Zhen Z, Chan CW, Lam KS, Tse HF, Yiu KH. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:22.
Hamada S, Schroeder J, Hoffmann R, Altiok E, Keszei A, Almalla M, Napp A, Marx N, Becker M. Prediction of outcomes in patients with chronic ischemic cardiomyopathy by layer-specific strain echocardiography: a proof of concept. J Am Soc Echocardiogr. 2016;29(5):412–20.
Tadic M, Cuspidi C, Vukomanovic V, Ilic S, Obert P, Kocijancic V, Celic V. Layer-specific deformation of the left ventricle in uncomplicated patients with type 2 diabetes and arterial hypertension. Arch Cardiovasc Dis. 2018;111(1):17–24.
Jørgensen PG, Jensen MT, Biering-Sørensen T, Mogelvang R, Fritz-Hansen T, Vilsbøll T, Rossing P, Jensen JS. Burden of uncontrolled metabolic risk factors and left ventricular structure and function in patients with type 2 diabetes mellitus. J Am Heart Assoc. 2018;7(19):e008856.
Nielsen R, Nørrelund H, Kampmann U, Bøtker HE, Møller N, Wiggers H. Effect of acute hyperglycemia on left ventricular contractile function in diabetic patients with and without heart failure: two randomized cross-over studies. PLoS ONE. 2013;8(1):e53247.
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34.
Frati AC, Rivera C, Espinoza M, Ariza CR, Diaz ME. Influence of acute hyperglycemia on left ventricular function in diabetics assessed by echocardiography. Clin Cardiol. 1987;10:594–7.
Xie J, Cui K, Hao H, Zhang Y, Lin H, Chen Z, Huang X, Cao S, Liao W, Bin J, Kitakaze M, Liao Y. Acute hyperglycemia suppresses left ventricular diastolic function and inhibits autophagic flux in mice under prohypertrophic stimulation. Cardiovasc Diabetol. 2016;15:136.
Tang WH, Cheng WT, Kravtsov GM, Tong XY, Hou XY, Chung SK, Chung SS. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am J Physiol Cell Physiol. 2010;299(3):C643–C653653.
Leung M, Wong VW, Hudson M, Leung DY. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ Cardiovasc Imaging. 2016;9(3):e003643.
Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The, "metabolic memory" theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients. 2017;9(5):437.
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003; 348, 2294–303.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Acknowledgements
Not applicable
Funding
The present study was supported by Ministry of Sciences, Republic of Serbia (Project Number 175097).
Author information
Authors and Affiliations
Contributions
KL, JB and MA wrote the manuscript, KL, JB, MA, NML and GK design the study, analyze the data and review the manuscript, GK performed echocardiography testing, JB, AJ, NR, LjS, IR, DP and LjB contribute to the acquisition of data and performed research, JM performed statistical analysis, SS performed biochemistry analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local Ethics Committee of the Faculty of Medicine University of Belgrade. Informed consent was obtained from all investigated subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bogdanović, J., Ašanin, M., Krljanac, G. et al. Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: an analysis based on two-dimensional speckle tracking echocardiography. Cardiovasc Diabetol 18, 68 (2019). https://doi.org/10.1186/s12933-019-0876-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-019-0876-3