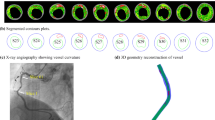
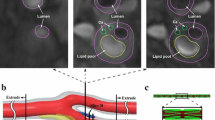
Abstract
Plaque haemorrhage (PH) in atherosclerotic plaques is associated with recurrent thromboembolic ischaemic events. The healing process predominantly involves the repair of the plaque rupture site and the replacement of fresh PH with chronic PH, which is either reabsorbed or replaced by fibrous tissue. The extent to which the presence of PH, and its type i.e. fresh or chronic, affects plaque stability remains unexplored. Finite element analysis (FEA)-based biomechanical stress simulations can provide quantification of the percentage contribution of PH and its types to the biomechanical stresses of plaques, thereby providing information about its role in plaque stability. Fifty-two patients with atherosclerotic carotid disease underwent high resolution magnetic resonance (MR) imaging of their carotid arteries in a 1.5 Tesla MR system. Twenty-three patients had MR-identifiable PH and were selected. Only those images of these patients were used for simulations, which had evidence of PH. Manual segmentation of plaque components, such as lipid pool, fibrous tissue, calcium and PH, was done using carotid MR images. Plaque components and vessel wall were modelled as isotropic, incompressible hyperelastic materials with non-linear properties undergoing deformation under patient-specific blood pressure loading. Two dimensional structure-only FEA was used for quantification of maximum critical stress (M-CStress) of plaques. The median M-CStress of symptomatic patients with fresh PH was 159 kPa (IQR: 114–253). Because PH usually occurs within the lipid pool, when the simulation was repeated with lipid pool replacing fresh PH to simulate the pre-rupture plaque state, M-CStress was reduced by 26% [118 kPa (IQR: 79–189) (P = 0.001)]. When fresh PH was replaced with chronic PH it resulted in a 30% reduction in the M-CStress [118 kP (IQR: 79–189), (P = 0.001)]. PH affects stresses within atheroma to various degrees depending on its type, thereby influencing plaque stability to a different extent, with fresh PH significantly increasing the biomechanical stresses. Plaque component-dependent stress analysis has the potential of identifying the critical nature of various plaque components.

Similar content being viewed by others
References
Chandratheva A, Mehta Z, Geraghty OC, Marquardt L, Rothwell PM (2009) Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology 72:1941–1947
Saam T, Yuan C, Chu B, Takaya N, Underhill H, Cai J, Tran N, Polissar NL, Neradilek B, Jarvik GP, Isaac C, Garden GA, Maravilla KR, Hashimoto B, Hatsukami TS (2007) Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis 194:e34–e42
Li ZY, Howarth S, Trivedi RA, U-King-Im JM, Graves MJ, Brown A, Wang L, Gillard JH (2006) Stress analysis of carotid plaque rupture based on in vivo high resolution MRI. J Biomech 39(14):2611–2622
Li ZY, Howarth S, Tang T, Graves M, U-King-Im JM, Gillard JH (2007) Does calcium deposition play a role in the stability of atheroma? Location may be the key. Cerebrovasc Dis 24:452–459
Li ZY, Howarth S, Trivedi RA, U-King-Im JM, Graves MJ, Brown A, Wang L, Gillard JH (2006) Stress analysis of carotid plaque rupture based on in vivo high resolution MRI. J Biomech 39:2611–2622
Li ZY, Howarth SP, Tang T, Gillard JH (2006) How critical is fibrous cap thickness to carotid plaque stability? A flow-plaque interaction model. Stroke 37:1195–1199
Barger AC, Beeuwkes R III, Lainey LL, Silverman KJ (1984) Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med 310:175–177
Sadat U, Teng Z, Young VE, Graves MJ, Gillard JH (2010) Three-dimensional volumetric analysis of atherosclerotic plaques: a magnetic resonance imaging-based study of patients with moderate stenosis carotid artery disease. Int J Cardiovasc Imaging. [Epub ahead of print]
Sadat U, Li ZY, Young VE, Graves MJ, Boyle JR, Warburton EL, Varty K, O’Brien E, Gillard JH (2010) Finite element analysis of vulnerable atherosclerotic plaques: a comparison of mechanical stresses within carotid plaques of acute and recently symptomatic patients with carotid artery disease. J Neurol Neurosurg Psychiatry 81(3):286–289
Sadat U, Weerakkody RA, Bowden DJ, Young VE, Graves MJ, Li ZY, Tang TY, Gaunt ME, Hayes PD, Gillard JH (2009) Utility of high resolution MR imaging to assess carotid plaque morphology: A comparison of acute symptomatic, recently symptomatic and asymptomatic patients with carotid artery disease. Atherosclerosis 207(2):434–439
Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS (2001) In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 104:2051–2056
Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O’Brien KD, Polissar NL, Hatsukami TS, Yuan C (2004) Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35:1079–1084
Yang C, Tang D, Yuan C, Hatsukami TS, Zheng J, Woodard PK (2007) In Vivo/Ex Vivo MRI-based 3D Non-Newtonian FSI models for human atherosclerotic plaques compared with fluid/wall-only models. Comput Model Eng Sci 19:233–246
Bathe KJ (2002) Theory and modeling guide vol I: ADINA; vol II: ADINA-F. ADINA R&D, Inc., Watertown
Teng Z, Sadat U, Li Z, Huang X, Zhu C, Young VE, Graves MJ, Gillard JH (2010) Arterial luminal curvature and fibrous-cap thickness affect critical stress conditions within atherosclerotic plaque: an in vivo MRI-based 2D finite-element study. Ann Biomed Eng. [Epub ahead of print]
Tang D, Yang C, Zheng J, Woodard PK, Saffitz JE, Petruccelli JD, Sicard GA, Yuan C (2005) Local maximal stress hypothesis and computational plaque vulnerability index for atherosclerotic plaque assessment. Ann Biomed Eng 33:1789–1801
Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R (2003) Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349:2316–2325
Versluis A, Bank AJ, Douglas WH (2006) Fatigue and plaque rupture in myocardial infarction. J Biomech 39:339–347
Couffinhal T, Kearney M, Witzenbichler B, Chen D, Murohara T, Losordo DW, Symes J, Isner JM (1997) Vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in normal and atherosclerotic human arteries. Am J Pathol 150:1673–1685
Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD (2001) Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 7:425–429
Department of Health (2007) National stroke strategy
Naylor AR (2008) Occam’s razor: intervene early to prevent more strokes!. J Vasc Surg 48:1053–1059
Kampschulte A, Ferguson MS, Kerwin WS, Polissar NL, Chu B, Saam T, Hatsukami TS, Yuan C (2004) Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation 110:3239–3244
Choi HY, Ye BS, Ahn SH, Cho HJ, Kim DJ, Kim SM, Heo JH (2009) Characteristics and the fate of intraluminal thrombus of the intracranial and extracranial cerebral arteries in acute ischemic stroke patients. Eur Neurol 62:72–78
Bhatti AF, Leon LR Jr, Labropoulos N, Rubinas TL, Rodriguez H, Kalman PG, Schneck M, Psalms SB, Biller J (2007) Free-floating thrombus of the carotid artery: literature review and case reports. J Vasc Surg 45:199–205
Gonzalez A, Mayol A, Gil-Peralta A, Gonzalez-Marcos JR, Boza F, Ruano J (2004) Angioplasty of symptomatic high-grade internal carotid artery stenosis with intraluminal thrombus: therapeutic approach. Neuroradiology 46:313–317
Biller J, Adams HP Jr, Boarini D, Godersky JC, Smoker WR, Kongable G (1986) Intraluminal clot of the carotid artery. A clinical-angiographic correlation of nine patients and literature review. Surg Neurol 25:467–477
Levine SR, Quint DJ, Pessin MS, Boulos RS, Welch KM (1989) Intraluminal clot in the vertebrobasilar circulation: clinical and radiologic features. Neurology 39:515–522
Pelz DM, Buchan A, Fox AJ, Barnett HJ, Vinuela F (1986) Intraluminal thrombus of the internal carotid arteries: angiographic demonstration of resolution with anticoagulant therapy alone. Radiology 160:369–373
Li ZY, Howarth SP, Tang T, Graves MJ, U-King-Im JM, Trivedi RA, Kirkpatrick PJ, Gillard JH (2007) Structural analysis and magnetic resonance imaging predict plaque vulnerability: a study comparing symptomatic and asymptomatic individuals. J Vasc Surg 45:768–775
Sadat U, Teng Z, Young VE, Walsh SR, Li ZY, Graves MJ, Varty K, Gillard JH (2010) Association between biomechanical structural stresses and morphological characteristics of atherosclerotic carotid plaques and subsequent ischaemic cerebrovascular events—a longitudinal in vivo MRI-based finite-element study. Eur J Vasc Endovasc Surg (in press)
Trivedi RA, U-King-Im JM, Graves MJ, Horsley J, Goddard M, Kirkpatrick PJ, Gillard JH (2004) MRI-derived measurements of fibrous-cap and lipid-core thickness: the potential for identifying vulnerable carotid plaques in vivo. Neuroradiology 46:738–743
Acknowledgments
Dr. Umar Sadat is supported by a Medical Research Council UK & Royal College of Surgeons of England Joint Clinical Research Training Fellowship. This research has also been supported by a Biomedical Research Centre National Institute of Health Research (BRC NIHR) grant.
Competing interests
None.
Declaration
Umar Sadat and Zhongzhao Teng have equal contribution in writing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadat, U., Teng, Z., Young, V.E. et al. Impact of plaque haemorrhage and its age on structural stresses in atherosclerotic plaques of patients with carotid artery disease: an MR imaging-based finite element simulation study. Int J Cardiovasc Imaging 27, 397–402 (2011). https://doi.org/10.1007/s10554-010-9679-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-010-9679-z