Abstract
Purpose
Previous literature shows that more bladder cancer patients overall die from causes other than the primary malignancy. Given known disparities in bladder cancer outcomes by race and sex, we aimed to characterize differences in cause-specific mortality for bladder cancer patients by these demographics.
Methods
We identified 215,252 bladder cancer patients diagnosed with bladder cancer from 2000 to 2017 in the SEER 18 database. We calculated cumulative incidence of death from seven causes (bladder cancer, COPD, diabetes, heart disease, external, other cancer, other) to assess differences in cause-specific mortality between race and sex subgroups. We used multivariable Cox proportional hazards regression and Fine-Gray competing risk models to compare risk of bladder cancer-specific mortality between race and sex subgroups overall and stratified by cancer stage.
Results
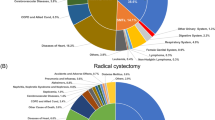
17% of patients died from bladder cancer (n = 36,923), 30% died from other causes (n = 65,076), and 53% were alive (n = 113,253). Among those who died, the most common cause of death was bladder cancer, followed by other cancer and diseases of the heart. All race-sex subgroups were more likely than white men to die from bladder cancer. Compared to white men, white women (HR: 1.20, 95% CI: 1.17–1.23) and Black women (HR: 1.57, 95% CI: 1.49–1.66) had a higher risk of dying from bladder cancer, overall and stratified by stage.
Conclusion
Among bladder cancer patients, death from other causes especially other cancer and heart disease contributed a large proportion of mortality. We found differences in cause-specific mortality by race-sex subgroups, with Black women having a particularly high risk of dying from bladder cancer.

Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available in the Open Science Framework repository, https://osf.io/u9mty/. These data were derived from the following resource available in the public domain: Surveillance Research Program, National Cancer Institute SEER*Stat software version 8.4.0.1 [48].
Abbreviations
- SEER:
-
Surveillance, Epidemiology, and End Results
- COPD:
-
Chronic obstructive pulmonary disease
- HR:
-
Hazard ratio
- CI:
-
95% Confidence interval
References
American Cancer Society (2021) Cancer Facts & Figures 2021. ACS, Atlanta
Cárdenas-Turanzas M, Cooksley C, Pettaway CA et al (2006) Comparative outcomes of bladder cancer. Obstet Gynecol 108:169–175. https://doi.org/10.1097/01.AOG.0000223885.25192.91
Mungan NA, Aben KK, Schoenberg MP et al (2000) Gender differences in stage-adjusted bladder cancer survival. Urology 55:876–880. https://doi.org/10.1016/s0090-4295(00)00523-9
Scosyrev E, Noyes K, Feng C, Messing E (2009) Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 115:68–74. https://doi.org/10.1002/cncr.23986
Zhai M, Tang C, Li M et al (2020) Short-term mortality risks among patients with non-metastatic bladder cancer. BMC Cancer 20:1148. https://doi.org/10.1186/s12885-020-07655-x
Wang W, Liu J, Liu L (2021) Development and validation of a prognostic model for predicting overall survival in patients with bladder cancer: a SEER-based study. Front Oncol 11:692728. https://doi.org/10.3389/fonc.2021.692728
Ballas LK, Navarro S, Luo C et al (2021) Disparities in male versus female oncologic outcomes following bladder preservation: a population-based cohort study. Cancer Med 10:3004–3012. https://doi.org/10.1002/cam4.3835
Kaye DR, Canner JK, Kates M et al (2016) Do African American patients treated with radical cystectomy for bladder cancer have worse overall survival? accounting for pathologic staging and patient demographics beyond race makes a difference. Bladder Cancer 2:225–234. https://doi.org/10.3233/BLC-150041
Bhambhvani HP, Zamora A, Shkolyar E et al (2021) Development of robust artificial neural networks for prediction of 5-year survival in bladder cancer. Urol Oncol 39:193.e7-193.e12. https://doi.org/10.1016/j.urolonc.2020.05.009
Wang Y, Chang Q, Li Y (2018) Racial differences in urinary bladder cancer in the United States. Sci Rep. https://doi.org/10.1038/s41598-018-29987-2
Rosiello G, Knipper S, Palumbo C et al (2020) Rates of other-cause mortality after radical cystectomy are decreasing over time—a population-based analysis over two decades. J Surg Oncol 121:1329–1336. https://doi.org/10.1002/jso.25919
Bukavina L, Prunty M, Mishra K et al (2020) Gender disparities in bladder cancer-specific survival in high poverty areas utilizing ohio cancer incidence surveillance system (OCISS). Urology. https://doi.org/10.1016/j.urology.2020.07.013
Noon AP, Albertsen PC, Thomas F et al (2013) Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer 108:1534–1540. https://doi.org/10.1038/bjc.2013.106
Registry Groupings in SEER Data and Statistics - SEER Registries. In: SEER. https://seer.cancer.gov/registries/terms.html. Accessed 19 Dec 2021
National Cancer Institute (2021) About the SEER Program. In: SEER. https://seer.cancer.gov/about/index.html. Accessed 11 May 2021
World Health Organization (2021) Classification of Diseases (ICD). In: WHO. https://www.who.int/standards/classifications/classification-of-diseases. Accessed 11 May 2021
National Cancer Institute (2018) SEER Cause of Death Recode 1969+ (03/01/2018) - SEER Data Reporting Tools. In: SEER. https://seer.cancer.gov/codrecode/1969_d03012018/index.html. Accessed 11 May 2021
US Department of Health and Human Services (2020) Smoking Cessation: A Report of the Surgeon General. HHS, Washington
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509. https://doi.org/10.1080/01621459.1999.10474144
Fang W, Yang Z-Y, Chen T-Y et al (2020) Ethnicity and survival in bladder cancer: a population-based study based on the SEER database. J Transl Med 18:145. https://doi.org/10.1186/s12967-020-02308-w
Lee CT, Dunn RL, Williams C, Underwood W (2006) Racial disparity in bladder cancer: trends in tumor presentation at diagnosis. J Urol 176:927–933. https://doi.org/10.1016/j.juro.2006.04.074
Danforth KN, Luong TQ, Yi DK et al (2020) Disparities in stage at diagnosis in an equal-access integrated delivery system: a retrospective cohort study of 7244 patients with bladder cancer. Clin Genitourin Cancer 18:e91–e102. https://doi.org/10.1016/j.clgc.2019.09.002
Radkiewicz C, Edgren G, Johansson ALV et al (2020) Sex Differences in urothelial bladder cancer survival. Clin Genitourin Cancer 18:26-34.e6. https://doi.org/10.1016/j.clgc.2019.10.020
Andreassen BK, Grimsrud TK, Haug ES (2018) Bladder cancer survival: women better off in the long run. Eur J Cancer 95:52–58. https://doi.org/10.1016/j.ejca.2018.03.001
Washington SL, Neuhaus J, Meng MV, Porten SP (2019) Social determinants of appropriate treatment for muscle-invasive bladder cancer. Cancer Epidemiol Biomarkers Prev 28:1339–1344. https://doi.org/10.1158/1055-9965.EPI-18-1280
Schrag D, Hsieh LJ, Rabbani F et al (2003) Adherence to surveillance among patients with superficial bladder cancer. J Natl Cancer Inst 95:588–597. https://doi.org/10.1093/jnci/95.8.588
Williams SB, Huo J, Kosarek CD et al (2017) Population-based assessment of racial/ethnic differences in utilization of radical cystectomy for patients diagnosed with bladder cancer. Cancer Causes Control 28:755–766. https://doi.org/10.1007/s10552-017-0902-2
Chase DM, Fedewa S, Chou TS et al (2012) Disparities in the allocation of treatment in advanced ovarian cancer: are there certain patient characteristics associated with nonstandard therapy? Obstet Gynecol 119:68–77. https://doi.org/10.1097/AOG.0b013e31823d4006
Liu F, Randall L, Tewari K, Bristow R (2014) Racial disparities and patterns of ovarian cancer surgical care in California. Gynecol Oncol 132:221–226. https://doi.org/10.1016/j.ygyno.2013.08.035
Newman LA, Kaljee LM (2017) Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg 152:485–493. https://doi.org/10.1001/jamasurg.2017.0005
Robinson JC (1989) Exposure to occupational hazards among Hispanics, blacks and non-Hispanic whites in California. Am J Public Health 79:629–630
Khanal A, Budhathoki N, Singh VP, Shah BK (2017) Second primary malignancy in bladder carcinoma - a population-based Study. Anticancer Res 37:2033–2036. https://doi.org/10.21873/anticanres.11548
Kwon W-A, Joung JY, Lim J et al (2018) Risk of second primary Cancer among bladder Cancer patients: a population-based cohort study in Korea. BMC Cancer 18:617. https://doi.org/10.1186/s12885-018-4530-3
Rink M, Zabor EC, Furberg H et al (2013) Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. Eur Urol 64:456–464. https://doi.org/10.1016/j.eururo.2012.11.039
Shiels MS, Gibson T, Sampson J et al (2014) Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage i lung cancers. J Clin Oncol 32:3989–3995. https://doi.org/10.1200/JCO.2014.56.8220
Sturgeon KM, Deng L, Bluethmann SM et al (2019) A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 40:3889–3897. https://doi.org/10.1093/eurheartj/ehz766
Kong J, Diao X, Diao F et al (2019) Causes of death in long-term bladder cancer survivors: a population-based study. Asia Pac J Clin Oncol 15:e167–e174. https://doi.org/10.1111/ajco.13156
Stoltzfus KC, Zhang Y, Sturgeon K et al (2020) Fatal heart disease among cancer patients. Nat Commun 11:2011. https://doi.org/10.1038/s41467-020-15639-5
Zaorsky NG, Churilla TM, Egleston BL et al (2017) Causes of death among cancer patients. Ann Oncol 28:400–407. https://doi.org/10.1093/annonc/mdw604
Yu J, Lim B, Lee Y et al (2020) Risk factors and outcomes of myocardial injury after non-cardiac surgery in high-risk patients who underwent radical cystectomy. Medicine (Baltimore) 99:e22893. https://doi.org/10.1097/MD.0000000000022893
Jun I-J, Kim J, Kim H-G et al (2019) Risk factors of postoperative major adverse cardiac events after radical cystectomy: implication of diastolic dysfunction. Sci Rep 9:14096. https://doi.org/10.1038/s41598-019-50582-6
Baser S, Shannon VR, Eapen GA et al (2006) Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest 130:1784–1790. https://doi.org/10.1378/chest.130.6.1784
Simonis K, Shariat SF, Rink M, Urothelial Cancer Working Group of the Young Academic Urologists (YAU), Working Party of the European Association of Urology (EAU) (2014) Smoking and smoking cessation effects on oncological outcomes in nonmuscle invasive bladder cancer. Curr Opin Urol 24:492–499. https://doi.org/10.1097/MOU.0000000000000079
Zeegers MP, Tan FE, Dorant E, van Den Brandt PA (2000) The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer 89:630–639. https://doi.org/10.1002/1097-0142(20000801)89:3%3c630::aid-cncr19%3e3.3.co;2-h
Qin Q, Xu X, Wang X, Zheng X-Y (2013) Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev 14:3117–3121. https://doi.org/10.7314/apjcp.2013.14.5.3117
Sun J-W, Zhao L-G, Yang Y et al (2015) Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One 10:e0119313. https://doi.org/10.1371/journal.pone.0119313
Lu Y, Tao J (2021) Diabetes mellitus and obesity as risk factors for bladder cancer prognosis: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 12:699732. https://doi.org/10.3389/fendo.2021.699732
National Cancer Institute (2018) SEER*Stat Software. In: SEER. https://seer.cancer.gov/seerstat/index.html. Accessed 22 Jun 2022
Acknowledgments
We appreciate the contributions from cancer registries supported by the National Cancer Institute’s SEER program. This study was supported with funding from the National Cancer Institute (SPORE P20CA233216) and the Mt. Sinai Health Care Foundation.
Funding
This study was supported with funding from the Division of Cancer Epidemiology and Genetics, the National Cancer Institute (SPORE P20CA233216) and the Mt. Sinai Health Care Foundation.
Author information
Authors and Affiliations
Contributions
TDS contributed to data curation, formal analysis, investigation, and writing of the original draft. SCM contributed to conceptualization, funding acquisition, formal analysis, investigation, methodology, supervision, and writing, reviewing, and editing of the manuscript. FRS, BC, LP, AM, LB, and AC contributed to investigation and writing, reviewing, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was deemed not human subjects research by the Case Western Reserve University Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shu, T.D., Schumacher, F.R., Conroy, B. et al. Disparities in cause-specific mortality by race and sex among bladder cancer patients from the SEER database. Cancer Causes Control 34, 521–531 (2023). https://doi.org/10.1007/s10552-023-01679-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-023-01679-x