Abstract
Purpose
Endocrine therapy for breast cancer can exacerbate menopausal symptoms. The association between endocrine therapy and common pelvic floor disorders including urinary incontinence has rarely been evaluated. We examined urogenital and sexual side effects among women with a breast cancer diagnosis, comparing endocrine therapy users to nonusers.
Methods
Urogenital and sexual symptoms were self-reported during the enrollment interview within the University of North Carolina Cancer Survivorship Cohort. Tumor characteristics and endocrine therapy use were collected from medical and prescription records. We calculated multivariable prevalence ratios (PR) and 95 % confidence intervals (CI) for the association of endocrine therapy (versus no endocrine therapy) and urinary incontinence, overall and by therapy type (tamoxifen or aromatase inhibitors). PROMIS Sexual Function and Satisfaction domain scores were compared across endocrine therapy groups.
Results
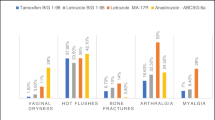
Among the 548 women with a breast cancer diagnosis, 49 % received endocrine therapy. Overall, 18 % of women reported urinary incontinence symptoms. We observed no association between urinary incontinence and endocrine therapy use overall (PR = 0.97; 95 % CI 0.67, 1.43), tamoxifen (PR = 1.20; 95 % CI 0.74, 1.96), or aromatase inhibitors (PR = 0.89; 95 % CI 0.55, 1.42), compared to no use. Approximately 55 % of women were sexually active. Sexual function scores did not vary according to endocrine therapy use, although urinary incontinence was associated with lower satisfaction scores (p = 0.05).
Conclusions
Our findings demonstrate a high prevalence of urinary incontinence after breast cancer diagnosis similar to the overall prevalence in older U.S. women, and this did not vary strongly according to use of endocrine therapy.

Similar content being viewed by others
References
American Cancer Society (2015) Cancer facts & figures 2015. American Cancer Society, Atlanta
Howlader N, Noone AM, Krapcho M et al (2015) SEER cancer statistics review, 1975–2012. National Cancer Institute, Bethesda
American Cancer Society (2015) Breast cancer facts & figures 2015–2016. American Cancer Society, Inc., Atlanta
Burstein HJ, Temin S, Anderson H et al (2014) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol 32(21):2255–2269. doi:10.1200/jco.2013.54.2258
Chin SN, Trinkaus M, Simmons C et al (2009) Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 9(2):108–117. doi:10.3816/CBC.2009.n.020
Cella D, Fallowfield L, Barker P et al (2006) Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 100(3):273–284. doi:10.1007/s10549-006-9260-6
Fallowfield LJ, Bliss JM, Porter LS et al (2006) Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol 24(6):910–917. doi:10.1200/jco.2005.03.3654
Morales L, Neven P, Timmerman D et al (2004) Acute effects of tamoxifen and third-generation aromatase inhibitors on menopausal symptoms of breast cancer patients. Anticancer Drugs 15(8):753–760
Nygaard I, Barber MD, Burgio KL et al (2008) Prevalence of symptomatic pelvic floor disorders in US women. JAMA 300(11):1311–1316. doi:10.1001/jama.300.11.1311
Wu JM, Matthews CA, Vaughan CP et al (2015) Urinary, fecal, and dual incontinence in older U.S. adults. J Am Geriatr Soc 63(5):947–953. doi:10.1111/jgs.13385
White AJ, Reeve BB, Chen RC et al (2013) Urinary incontinence and health-related quality of life among older Americans with and without cancer: a cross-sectional study. BMC Cancer 13:377. doi:10.1186/1471-2407-13-377
Albertazzi P, Sharma S (2005) Urogenital effects of selective estrogen receptor modulators: a systematic review. Climacteric 8(3):214–220. doi:10.1080/13697130500117946
Hendrix SL, Cochrane BB, Nygaard IE et al (2005) Effects of estrogen with and without progestin on urinary incontinence. JAMA 293(8):935–948. doi:10.1001/jama.293.8.935
Sharma S, Albertazzi P, Bottazzi M (2007) The long-term effect of raloxifene on the genitourinary tract. Climacteric 10(3):244–248. doi:10.1080/13697130701379311
Zbucka-Kretowska M, Marcus-Braun N, Eboue C et al (2011) Expression of estrogen receptors in the pelvic floor of pre- and post-menopausal women presenting pelvic organ prolapse. Folia Histochem Cytobiol 49(3):521–527
Flynn KE, Lin L, Cyranowski JM et al (2013) Development of the NIH PROMIS (R) sexual function and satisfaction measures in patients with cancer. J Sex Med 10(Suppl 1):43–52. doi:10.1111/j.1743-6109.2012.02995.x
PROMIS Sexual Function Domain Group (2015) PROMIS Sexual Function and Satisfaction Measures User Manual. PROMIS Network, Chapel Hill, NC. http://assessmentcenter.net/documents/Sexual%20Function%20Manual.pdf. Accessed 20 Dec 2015
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41(5):582–592. doi:10.1097/01.mlr.0000062554.74615.4c
Baumgart J, Nilsson K, Stavreus-Evers A et al (2011) Urogenital disorders in women with adjuvant endocrine therapy after early breast cancer. Am J Obstet Gynecol 204(1):26 e1–26 e7. doi:10.1016/j.ajog.2010.08.035
Cody JD, Jacobs ML, Richardson K et al (2012) Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev 10:CD001405. doi:10.1002/14651858.CD001405.pub3
Agency for Healthcare Research and Quality (2012) Nonsurgical treatments for urinary incontinence in adult women: diagnosis and comparative effectiveness. Agency for Healthcare Research and Quality, Department of Health and Human Services, Rockville, MD, Contract No.: Pub. No. 11(12)-EHC074-1
Buzdar A, Howell A, Cuzick J et al (2006) Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 7(8):633–643. doi:10.1016/s1470-2045(06)70767-7
Frechette D, Paquet L, Verma S et al (2013) The impact of endocrine therapy on sexual dysfunction in postmenopausal women with early stage breast cancer: encouraging results from a prospective study. Breast Cancer Res Treat 141(1):111–117. doi:10.1007/s10549-013-2659-y
Acknowledgments
The authors thank the UNC Health Registry/Cancer Survivorship Cohort (HR/CSC) participants for their important contributions and appreciate the helpful comments of Dr. Antonia Bennett. The UNC HR/CSC is funded in part by the UNC Lineberger Comprehensive Cancer Center’s University Cancer Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported in part by the National Center for Advancing Translational Sciences (KL2-TR001109).
Conflict of interest
Dr. Jennifer Wu has received institutional research grants from Boston Scientific and Pelvalon. The remaining authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Landi, S.N., Doll, K.M., Bensen, J.T. et al. Endocrine therapy and urogenital outcomes among women with a breast cancer diagnosis. Cancer Causes Control 27, 1325–1332 (2016). https://doi.org/10.1007/s10552-016-0810-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-016-0810-x