Abstract
Purpose
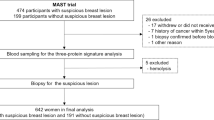
To investigate the combined use of blood-based 3-protein signature and breast ultrasound (US) for validating US-detected lesions.
Methods
From July 2011 to April 2020, women who underwent whole-breast US within at least 6 months from sampling period were retrospectively included. Blood-based 3-protein signature (Mastocheck®) value and US findings were evaluated. Following outcome measures were compared between US alone and the combination of Mastocheck® value with US: sensitivity, specificity, positive predictive value (PPV), negative predictive value, area under the receiver operating characteristic curve (AUC), and biopsy rate.
Results
Among the 237 women included, 59 (24.9%) were healthy individuals and 178 (75.1%) cancer patients. Mean size of cancers was 1.2 ± 0.8 cm. Median value of Mastocheck® was significantly different between nonmalignant (− 0.24, interquartile range [IQR] − 0.48, − 0.03) and malignant lesions (0.55, IQR − 0.03, 1.42) (P < .001). Utilizing Mastocheck® value with US increased the AUC from 0.67 (95% confidence interval [CI] 0.61, 0.73) to 0.81 (95% CI 0.75, 0.88; P < .001), and specificity from 35.6 (95% CI 23.4, 47.8) to 64.4% (95% CI 52.2, 76.6; P < .001) without loss in sensitivity. PPV was increased from 82.2 (95% CI 77.1, 87.3) to 89.3% (95% CI 85.0, 93.6; P < .001), and biopsy rate was significantly decreased from 79.3 (188/237) to 72.1% (171/237) (P < .001). Consistent improvements in specificity, PPV, and AUC were observed in asymptomatic women, in women with dense breast, and in those with normal/benign mammographic findings.
Conclusion
Mastocheck® is an effective tool that can be used with US to improve diagnostic specificity and reduce false-positive findings and unnecessary biopsies.





Similar content being viewed by others
References
Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, Törnberg S, Chen SL, Chiu SY, Fann JC, Ku MM, Wu WY, Hsu CY, Chen YC, Svane G, Azavedo E, Grundström H, Sundén P, Leifland K, Frodis E, Ramos J, Epstein B, Åkerlund A, Sundbom A, Bordás P, Wallin H, Starck L, Björkgren A, Carlson S, Fredriksson I, Ahlgren J, Öhman D, Holmberg L, Chen TH (2020) Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer 126:2971–2979. https://doi.org/10.1002/cncr.32859
Tabár L, Dean PB, Chen TH, Yen AM, Chen SL, Fann JC, Chiu SY, Ku MM, Wu WY, Hsu CY, Chen YC, Beckmann K, Smith RA, Duffy SW (2019) The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 125:515–523. https://doi.org/10.1002/cncr.31840
Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, White E (2000) Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 92:1081–1087. https://doi.org/10.1093/jnci/92.13.1081
Crystal P, Strano SD, Shcharynski S, Koretz MJ (2003) Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol 181:177–182. https://doi.org/10.2214/ajr.181.1.1810177
Kaplan SS (2001) Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology 221:641–649. https://doi.org/10.1148/radiol.2213010364
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225:165–175. https://doi.org/10.1148/radiol.2251011667
Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, Pisano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Larsen LH, Barr RG, Farria DM, Marques HS, Boparai K (2008) Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 299:2151–2163. https://doi.org/10.1001/jama.299.18.2151
Corsetti V, Ferrari A, Ghirardi M, Bergonzini R, Bellarosa S, Angelini O, Bani C, Ciatto S (2006) Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med 111:440–448. https://doi.org/10.1007/s11547-006-0040-5
Tohno E, Ueno E, Watanabe H (2009) Ultrasound screening of breast cancer. Breast Cancer 16:18–22. https://doi.org/10.1007/s12282-008-0082-8
Bassett LW (2000) Imaging of breast masses. Radiol Clin North Am 38:669–691. https://doi.org/10.1016/s0033-8389(05)70193-7
Moy L, Slanetz PJ, Moore R, Satija S, Yeh ED, McCarthy KA, Hall D, Staffa M, Rafferty EA, Halpern E, Kopans DB (2002) Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology 225:176–181. https://doi.org/10.1148/radiol.2251010999
Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, Cink TM, Coscia JL Jr, Eklund GW, Evans WP 3rd, Garver PR et al (1994) Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 193:359–364. https://doi.org/10.1148/radiology.193.2.7972743
Berg WA (2016) Current status of supplemental screening in dense breasts. J Clin Oncol 34:1840–1843. https://doi.org/10.1200/jco.2015.65.8674
Berg WA (2004) Supplemental screening sonography in dense breasts. Radiol Clin North Am 42:845–851. https://doi.org/10.1016/j.rcl.2004.04.003
Lee JM, Arao RF, Sprague BL, Kerlikowske K, Lehman CD, Smith RA, Henderson LM, Rauscher GH, Miglioretti DL (2019) Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk. JAMA Intern Med 179:658–667. https://doi.org/10.1001/jamainternmed.2018.8372
Corsetti V, Houssami N, Ferrari A, Ghirardi M, Bellarosa S, Angelini O, Bani C, Sardo P, Remida G, Galligioni E, Ciatto S (2008) Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur J Cancer 44:539–544. https://doi.org/10.1016/j.ejca.2008.01.009
Sprague BL, Stout NK, Schechter C, van Ravesteyn NT, Cevik M, Alagoz O, Lee CI, van den Broek JJ, Miglioretti DL, Mandelblatt JS, de Koning HJ, Kerlikowske K, Lehman CD, Tosteson AN (2015) Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med 162:157–166. https://doi.org/10.7326/m14-0692
Weigert JM (2017) The connecticut experiment; the third installment: 4 years of screening women with dense breasts with bilateral ultrasound. Breast J 23:34–39. https://doi.org/10.1111/tbj.12678
Underwood JJ, Quadri RS, Kalva SP, Shah H, Sanjeevaiah AR, Beg MS, Sutphin PD (2020) Liquid biopsy for cancer: review and implications for the radiologist. Radiology 294:5–17. https://doi.org/10.1148/radiol.2019182584
Ziegler A, Zangemeister-Wittke U, Stahel RA (2002) Circulating DNA: a new diagnostic gold mine? Cancer Treat Rev 28:255–271. https://doi.org/10.1016/s0305-7372(02)00077-4
Tay TKY, Tan PH (2020) Liquid biopsy in breast cancer: a focused review. Arch Pathol Lab Med 145:678–686. https://doi.org/10.5858/arpa.2019-0559-RA
Alimirzaie S, Bagherzadeh M, Akbari MR (2019) Liquid biopsy in breast cancer: a comprehensive review. Clin Genet 95:643–660. https://doi.org/10.1111/cge.13514
Loke SY, Lee ASG (2018) The future of blood-based biomarkers for the early detection of breast cancer. Eur J Cancer 92:54–68. https://doi.org/10.1016/j.ejca.2017.12.025
Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M (2016) Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev 35:347–376. https://doi.org/10.1007/s10555-016-9629-x
Chen L, Bode AM, Dong Z (2017) Circulating tumor cells: moving biological insights into detection. Theranostics 7:2606–2619. https://doi.org/10.7150/thno.18588
Li J, Guan X, Fan Z, Ching LM, Li Y, Wang X, Cao WM, Liu DX (2020) Non-invasive biomarkers for early detection of breast cancer. Cancers (Basel). https://doi.org/10.3390/cancers12102767
Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, Hruban RH, Wolfgang CL, Goggins MG, Dal Molin M, Wang TL, Roden R, Klein AP, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Vogelstein JT, Browne JD, Schoen RE, Brand RE, Tie J, Gibbs P, Wong HL, Mansfield AS, Jen J, Hanash SM, Falconi M, Allen PJ, Zhou S, Bettegowda C, Diaz LA Jr, Tomasetti C, Kinzler KW, Vogelstein B, Lennon AM, Papadopoulos N (2018) Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359:926–930. https://doi.org/10.1126/science.aar3247
Duffy MJ (2006) Serum tumor markers in breast cancer: are they of clinical value? Clin Chem 52:345–351. https://doi.org/10.1373/clinchem.2005.059832
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25:5287–5312. https://doi.org/10.1200/jco.2007.14.2364
Kazarian A, Blyuss O, Metodieva G, Gentry-Maharaj A, Ryan A, Kiseleva EM, Prytomanova OM, Jacobs IJ, Widschwendter M, Menon U, Timms JF (2017) Testing breast cancer serum biomarkers for early detection and prognosis in pre-diagnosis samples. Br J Cancer 116:501–508. https://doi.org/10.1038/bjc.2016.433
Kim Y, Kang UB, Kim S, Lee HB, Moon HG, Han W, Noh DY (2019) A validation study of a multiple reaction monitoring-based proteomic assay to diagnose breast cancer. J Breast Cancer 22:579–586. https://doi.org/10.4048/jbc.2019.22.e57
Kim Y, Moon H-G, Lee H-B, Moon WK, Cho N, Chang J-M, Han W, Noh D-Y (2019) Efficacy of Mastocheck for screening of early breast cancer: comparison with screening mammography. J Breast Dis 7:59–64. https://doi.org/10.14449/jbd.2019.7.2.59
Lee HB, Kang UB, Moon HG, Lee J, Lee KM, Yi M, Park YS, Lee JW, Yu JH, Choi SH, Cho SH, Lee C, Han W, Noh DY (2015) Development and validation of a novel plasma protein signature for breast cancer diagnosis by using multiple reaction monitoring-based mass spectrometry. Anticancer Res 35:6271–6279
Mendelson EBB-VM, Berg WA et al (2013) ACR BI-RADS ultrasound. ACR BIRADS atlas, breast imaging reporting and data system. American College of Radiology, Reston, VA
Kang UB, Ahn Y, Lee JW, Kim YH, Kim J, Yu MH, Noh DY, Lee C (2010) Differential profiling of breast cancer plasma proteome by isotope-coded affinity tagging method reveals biotinidase as a breast cancer biomarker. BMC Cancer 10:114. https://doi.org/10.1186/1471-2407-10-114
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Lee SH, Chung J, Choi HY, Choi SH, Ryu EB, Ko KH, Koo HR, Park JS, Yi A, Youk JH, Son EJ, Chu AJ, Chang JM, Cho N, Jang MJ, Kook SH, Cha ES, Moon WK (2017) Evaluation of screening US-detected breast masses by combined use of elastography and color doppler US with B-mode US in women with dense breasts: a multicenter prospective study. Radiology 285:660–669. https://doi.org/10.1148/radiol.2017162424
Barr RG (2019) Future of breast elastography. Ultrasonography 38:93–105. https://doi.org/10.14366/usg.18053
Choi JS, Han BK, Ko ES, Bae JM, Ko EY, Song SH, Kwon MR, Shin JH, Hahn SY (2019) Effect of a deep learning framework-based computer-aided diagnosis system on the diagnostic performance of radiologists in differentiating between malignant and benign masses on breast ultrasonography. Korean J Radiol 20:749–758. https://doi.org/10.3348/kjr.2018.0530
Choi JH, Kang BJ, Baek JE, Lee HS, Kim SH (2018) Application of computer-aided diagnosis in breast ultrasound interpretation: improvements in diagnostic performance according to reader experience. Ultrasonography 37:217–225. https://doi.org/10.14366/usg.17046
Kim S, Choi Y, Kim E, Han BK, Yoon JH, Choi JS, Chang JM (2021) Deep learning-based computer-aided diagnosis in screening breast ultrasound to reduce false-positive diagnoses. Sci Rep 11:395. https://doi.org/10.1038/s41598-020-79880-0
Altman DG, Bland JM (1994) Diagnostic tests 2: predictive values. BMJ 309:102. https://doi.org/10.1136/bmj.309.6947.102
Acknowledgements
We thank Hwa Jung Kim, MD, PhD, associate professor of preventive medicine, for helping us with the statistical analysis.
Funding
This study was supported by Seoul National University Hospital Research Fund (Grant No. 04-2021-0510).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hong-Kyu Kim and Yumi Kim have unlisted stocks of Berits Inc. Dong-Young Noh report conflict of interest as he became CEO of Berits Inc. since March 2021. Wonshik Han reports being a member on the board of directors of and holding stock and ownership interests at DCGen, Co., Ltd., not relevant to this study. Su Min Ha and Jung Min Chang have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This retrospective study was approved by the institutional review board, and the informed consent requirement was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2022_6527_MOESM1_ESM.jpg
Supplementary Fig. 1: Receiver Operating Characteristic curve for Mastocheck®, breast ultrasound, and Mastocheck® combined with breast ultrasound in 63 women with negative/benign mammography. The AUC of breast US (solid line) is 0.70 (95% confidence interval [CI]: 0.62, 0.77) and Mastocheck® (dashed line) is 0.67 (95% CI: 0.54, 0.81). AUC is increased to 0.84 (95% CI: 0.77, 0.91) (P < .001) by addition of Mastocheck® to breast ultrasound (dot-dashed line). (JPG 2309 KB)
10549_2022_6527_MOESM2_ESM.jpg
Supplementary Fig. 2: Receiver Operating Characteristic curve for Mastocheck®, breast ultrasound, and Mastocheck® combined with breast ultrasound in 135 asymptomatic women. The AUC of breast ultrasound (solid line) is 0.66 (95% confidence interval [CI]: 0.60, 0.73) and Mastocheck® (dashed line) is 0.75 (95% CI: 0.68, 0.82). AUC is increased to 0.81 (95% CI: 0.74, 0.88) (P <. 001) by addition of Mastocheck® to breast ultrasound (dot-dashed line). (JPG 2331 KB)
10549_2022_6527_MOESM3_ESM.jpg
Supplementary Fig. 3: Receiver Operating Characteristic curve for Mastocheck®, breast ultrasound, and Mastocheck® combined with breast ultrasound in 57 asymptomatic women with negative/benign mammography. The AUC of breast ultrasound (solid line) is 0.70 (95% confidence interval [CI]: 0.63, 0.78) and Mastocheck® (dashed line) is 0.70 (95% CI: 0.56, 0.85). AUC is increased to 0.85 (95% CI: 0.77, 0.92) (P < .001) by addition of Mastocheck® to breast ultrasound (dot-dashed line). (JPG 2332 KB)
Appendices
Appendix
Material and methods
Preparation of blood samples
Plasma samples were drawn from peripheral veins and were stored in tubes containing ethylene diaminetetra-acetic acid (EDTA) to prevent coagulation. Samples were transferred to the laboratory and underwent centrifugation at 1300×g for 10 min at 4 °C. The supernatant plasma was filtered through a cellulose acetate filter (0.2 μm pore site) and platelet-free plasma was stored at − 80 °C. For mass spectrometry (MS), plasma protein concentration was determined by the Bradford assay. The plasma protein samples were denatured by incubation in 50 mM Tris buffer (pH 8.0) containing 3 M urea at 37 °C for 30 min. Samples were reduced with 10 mM dithiothreitol for 1 h at 56 °C, treated with 60 mM iodoacetamide for 1 h at room temperature in the dark, and then diluted tenfold with 50 mM ammonium bicarbonate. Digestion was performed with sequencing-grade trypsin (Promega, Madison, WI, USA) at 37 °C overnight at protein: trypsin molar ratio of 50:1. Tryptic digests were desalted using a C18 SPE cartridge (Waters, Milford, MA, USA) and dried in vacuo. The dried samples were dissolved in 0.1% formic acid. One hundred femtomoles of a betagalactosidase (β-Gal) peptide (residues 954–962, GDFQFNISR) was added to the desalted peptide mixture.
Rights and permissions
About this article
Cite this article
Ha, S.M., Kim, HK., Kim, Y. et al. Diagnostic performance improvement with combined use of proteomics biomarker assay and breast ultrasound. Breast Cancer Res Treat 192, 541–552 (2022). https://doi.org/10.1007/s10549-022-06527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06527-1