Abstract
Background
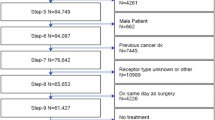
Neoadjuvant chemotherapy (NAC) is the standard of care for locally advanced HER2 + breast cancer (BC). Optimal sequencing of treatment (NAC vs. surgery first) is less clear cut in stage I (T1N0) HER2 + BC, where information from surgical pathology could impact adjuvant treatment decisions. Utilizing the NCDB, we evaluated the trend of NAC use compared to upfront surgery in patients with small HER2 + BC.
Methods
We identified NCDB female patients diagnosed with T1 N0 HER2 + BC from 2010 through 2015. Prevalence ratios (PR) using multivariable robust Poisson regression models were calculated to measure the association between baseline characteristics and the receipt of NAC. Analysis of trends over time was denoted by annual percent change (APC) of NAC versus surgery upfront.
Results
Of the 14,949 that received chemotherapy and anti-HER2 therapy during the study period, overall 1281 (8.6%) received NAC and 13,668 (91.4%) received adjuvant treatment. Patients receiving NAC increased annually from 4.2% in 2010 to 17.3% in 2015, with the most rapid increase occurring between years 2013 (8.5%) and 2014 (14.2%). The greatest increase was seen in patients with cT1c tumors with an APC of 37.8% over the study period (95% CI 29.0, 47.3%, p < 0.01), although a significant trend was likewise seen in patients with cT1a (APC = 26.1%,95% CI 1.59, 56.6%), and cT1b (APC = 27.4%, 95% CI 18.0, 37.7%) tumors. Predictors of neoadjuvant therapy receipt were age younger than 50 (PR = 1.69, 95% CI 1.52, 1.89), Mountain/Pacific area (PR = 1.24, 95% CI 1.05, 1.46), and estrogen receptor negativity (ER− PR + : PR = 2.01, 95% CI 1.51, 2.68; ER− PR− : PR = 1.49, 95% CI 1.32, 1.69).
Conclusions
Neoadjuvant therapy for T1 N0 HER2 + BC increased over the study period and was mostly due increased use in clinical T1c tumors. This may be consistent with secular change in Pertuzumab treatment following FDA approval in 2013.


Similar content being viewed by others
References
Perloff M, Lesnick GJ (1982) Chemotherapy before and after mastectomy in stage III breast cancer. Arch Surg 117:879
van der Hage JA, van de Velde C, Julien JP et al (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European organization for research and treatment of cancer trial 10902. J Clin Oncol 19(25):4224–4237
Nguyen TT, Hoskin TL, Day CN et al (2018) Decreasing use of axillary dissection in node-positive breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 25:2596–2602
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Fisher B, Brown A, Mamounas E et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15(7):2483–2493
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778
Murphy B, Day CN, Hoskin TL et al (2018) Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol 25:2241–2248
Puig C, Hoskin TL, Day CN et al (2017) National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a national cancer data base study. Ann Surg Oncol 24:1242–1250
Guangyong Z (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706
Tamhane AR, Westfall AO, Burkholder GA, Cutter GR (2016) Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med 35(30):5730–5735
Kim HJ, Fay MP, Feuer EJ, Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19:335–351
Slamon DJ, Godolphin W, Jones LA et al (1989) Studies of the HER2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712
Goldenberg MM (1999) Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther 21(2):309–318
Romond E, Suman V, Jeong J-H, et al (2012) Trastuzumab plus adjuvant chemotherapy for HER2-positive breast cancer: final planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. Paper presented at the San Antonio Breast Cancer Symposium
Slamon D, Eiermann W, Robert N et al (2011) Adjuvant Trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
Spielmann M, Roche H, Delozier T et al (2009) Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol 27(36):6129–6134
Joensuu H, Bono P, Kataja V et al (2009) Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 27(34):5685–5692
Gelber R, Goldhirsch A, Piccart M, et al (2012) HERA TRIAL: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years of median follow up. Paper presented at European Society of Medical Oncology, Vienna, Austria
Gianni L, Eiermann W, Semiglazov V et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomized controlled superiority trial with a parallel HER2-negative cohort. Lancet 375(9712):377–384
Gianni L, Pienkowski T, Im YH et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER-2 positive breast cancer (NeoSphere): a randomized multi-center, open-label, phase 2 trial. Lancet Oncol 13(1):25–32
Schneeweiss A, Chia S, Hickist T et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24(9):2278–2284
Mittendorf EA, Vila J, Tucker SL et al (2016) The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol 2(7):929–936
Fehrenbacher L, Capra A, Quesenberry C Jr et al (2014) Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol 32(20):2151–2158
Vaz-Luis I, Ottesen RA, Hughes ME et al (2014) Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol 32(20):2142–2150
Tolaney SM, Guo H, Pemas S et al (2019) Sever-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 37:1868–1875
Network NCC (2018) NCCN clinical practice guidelines in Oncology (NCCN Guidelines): Invasive breast cancer. National Comprehensive Cancer Network, Fort Washington
Slamon DJ, Eiermann W, Robert NJ et al (2016) Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res. 76:S5-04. https://doi.org/10.1158/1538-7445.SABCS15-S5-04
von Minckwitz G, Huang CS, Mano MS et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617–628
Acknowledgements
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
A waiver of informed consent was obtained as the information on the National Cancer Database is de-identified.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeidman, M., Schmidt, H., Alberty-Oller, J.J. et al. Trends in neoadjuvant chemotherapy versus surgery-first in stage I HER2-positive breast cancer patients in the National Cancer DataBase (NCDB). Breast Cancer Res Treat 187, 177–185 (2021). https://doi.org/10.1007/s10549-020-06041-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-06041-2