Abstract
Introduction
Neoadjuvant chemotherapy (NAC) is a well-established therapeutic option for patients with locally advanced disease often allowing downstaging and facilitation of breast conserving therapy. With evolution of better targeted treatment regimens and awareness of improved outcomes for significant responders, use of NAC has expanded particularly for triple negative and HER2-positive (HER2+) breast cancer. In this study, we explore utility of neoadjuvant chemotherapy for hormone receptor-positive HER2-negative (HR+ HER2−) patients.
Methods
Patients with HR+ HER2− breast cancer treated with chemotherapy before or after surgery were identified from 2010 to 2015 in the NCDB. Multivariable regression models adjusted for covariates were used to determine associations within these groups.
Results
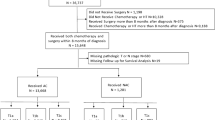
Among 134,574 patients (clinical stage 2A, 64%; 2B, 21%; 3, 15%), 105,324 (78%) had adjuvant chemotherapy (AC) and 29,250 (22%) received NAC. Use of NAC increased over time (2010–2015; 13.2–19.4% and PR = 1.34 for 2015; p < 0.0001). Patients were more likely to receive NAC with cT3, cT4, and cN+ disease. Patients less likely to receive NAC were age ≥ 50, lobular carcinoma, increased Charlson-Deyo score, and government insurance. Complete response (pCR) was noted in 8.3% of NAC patients. Axillary downstaging occurred in 21% of patients, and predictors included age < 50 years, black race, poorly differentiated grade, invasive ductal histology, and either ER or PR negativity.
Conclusions
NAC use among HR+ HER2− breast cancer patients has expanded over time and offers downstaging of disease for some patients, with pCR seen in only a small subset, but downstaging of the axilla in 21%. Further analysis is warranted to determine the subgroup of patients with HR+ HER2− disease who benefit from this approach.
Similar content being viewed by others
References
Perloff M, Lesnick GJ (1982) Chemotherapy before and after mastectomy in stage III breast cancer. Arch Surg 117:879
Fisher B, Brown A, Mamounas E et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15:2483
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778
Boughey JC, McCall LM, Ballman KV et al (2014) Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (alliance) prospective multicenter clinical trial. Ann Surg 260(4):608–616
Donker M, Straver ME, Wesseling J et al (2015) Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 261:378–382
Mamtani A, Barrio AV, King TA et al (2016) How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastasis? Results of a prospective study. Ann Surg Oncol 23:3467–3474
El Hage CH, Headon H, El Tokhy O et al (2016) Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3398 patients. Am J Surg 212:969
von Minckwitz G, Kummel S, Vogel P et al (2008) Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GerparTrio trial. J Natl Cancer Inst 100:542–551
von Minckwitz G, Blohmer JU, Costa SD et al (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31:3623–3630
Rigter LS, Loo CE, Linn SC et al (2013) Neoadjuvant chemotherapy adaptation and serial MRI response monitoring in ER-positive HER2-negative breast cancer. Br J Cancer 109:2965–2972
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366:2438–2441
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Anderson E (2002) The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4:197–201
Schott AF, Hayes DF (2012) Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol 30:1747
Murphy BL, Day CN, Hoskin TL et al (2018) Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol 25(8):2241–2248
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30:377–399
van der Heijden GJ, Donders AR, Stijnen T, Moons KG (2006) Imputation of missing values is superior to complete case analysis and the missing indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol 59:1102–1109
Guangyong Z (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706
Tamhane AR, Westfall AO, Burkholder GA, Cutter GR (2016) Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med 35(30):5730–5735
Esserman LJ, Berry DA, Cheang MC et al (2012) Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res 132:1049–1062
Kuerer HM, Newman LA, Buzdar AU et al (1998) Resideual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg 176:502–509
Spring LM, Fell G, Arfe A, et al (2018) Pathological complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival, stratified by breast cancer subtypes and adjuvant chemotherapy usage. San Antonio Breast Cancer Symposium. Presented December 5, 2018.
von Minckwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Fisher B, Jeong JH, Bryant J et al (2004) Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. Lancet 364:858–868
Puig C, Hoskin TL, Day CN et al (2017) National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a national cancer data base study. Ann Surg Oncol 24:1242–1250
Chiba A, Hoskin TL, Heins CN et al (2017) Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a national cancer data base study. Ann Surg Oncol 24:418–424
Ellis MJ, Suman VJ, Hoog J et al (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype-ACOSOG Z1031. J Clin Oncol 29(17):2342–2349
Suman VJ, Ellis EJ, Ma CX (2015) The alternate trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol 4(3):34
Chavez-MacGregor M, Litton J, Chen H et al (2010) Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy. Cancer 116:4168–4177
Warner ET, Ballman KV, Strand C et al (2016) Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426). Breast Cancer Res Treat 159:109–118
Killelea BK, Yang VQ, Wang SY et al (2015) Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol 33:4267–4275
Tichy JR, Deal AM, Anders CK et al (2015) Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat 150:667–674
Pastoriza JM, Karagiannis GS, Lin J et al (2018) Black race and distant recurrence after neoadjuvant or adjuvant chemotherapy in breast cancer. Clin Exp Metas 25:613–623
Acknowledgements
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All author declares that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeidman, M., Alberty-Oller, J.J., Ru, M. et al. Use of neoadjuvant versus adjuvant chemotherapy for hormone receptor-positive breast cancer: a National Cancer Database (NCDB) study. Breast Cancer Res Treat 184, 203–212 (2020). https://doi.org/10.1007/s10549-020-05809-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05809-w