Abstract
Background
Chemotherapy-induced amenorrhea (CIA) is one of the critical side effects from the chemotherapy in premenopausal patients with breast cancer. The goals of our study are the following: (1) to investigate the factors affecting the incidence of CIA; and (2) to evaluate the prognostic role of CIA in premenopausal patients with breast cancer.
Methods
We conducted a post hoc retrospective substudy to examine the incidence of the CIA and the relationship between CIA and prognosis in NSAS-BC02 that compared taxane alone to Doxorubicin(A) Cyclophosphamide(C) followed by taxane in postoperative patients with node-positive breast cancer
Results
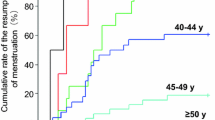
Of 395 premenopausal women, 287 (72.7%) had CIA due to protocol treatment. Regarding type of protocol regimen, proportion of CIA was 76.9% in AC Paclitaxel(P), 75.2% in AC Docetaxel(D), 62.8% in PTX, and 75.2% in DTX. Predictive factors of CIA were age increase by 5 years (OR 1.50), ER positivity (OR 2.08), and HER2 3 + ( OR 0.40) according to logistic regression analysis. According to the log rank test and the Cox proportional hazards model, CIA group had significantly better disease-free survival than non-CIA group (P < .0001). However, according to time-dependent Cox model that was used to reduce guarantee-time bias, CIA was not a statistically significant prognostic factor in both ER-positive and ER-negative patients.
Conclusion
Treatment with taxane alone caused high frequency of CIA in premenopausal women with breast cancer. CIA did not turn out to be an independent prognostic factor, taking guarantee-time bias into consideration. Further clinical studies are needed to validate these findings.

Similar content being viewed by others
References
Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C et al (1976) Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 294(8):405–410. https://doi.org/10.1056/NEJM197602192940801
Howard-Anderson J, Ganz PA, Bower JE, Stanton AL (2012) Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 104(5):386–405. https://doi.org/10.1093/jnci/djr541
Torino F, Barnabei A, De Vecchis L, Sini V, Schittulli F, Marchetti P et al (2014) Chemotherapy-induced ovarian toxicity in patients affected by endocrine-responsive early breast cancer. Crit Rev Oncol/Hematol 89(1):27–42. https://doi.org/10.1016/j.critrevonc.2013.07.007
Goldhirsch A, Gelber RD, Castiglione M (1990) The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients. The International Breast Cancer Study Group. Ann Oncol 1(3):183–188. https://doi.org/10.1093/oxfordjournals.annonc.a057718
Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N (1999) Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17(8):2365–2370. https://doi.org/10.1200/JCO.1999.17.8.2365
International Breast Cancer Study G, Basser RL, O'Neill A, Martinelli G, Green MD, Peccatori F et al (2006) Multicycle dose-intensive chemotherapy for women with high-risk primary breast cancer: results of International Breast Cancer Study Group Trial. J Clin Oncol 24(3):370–378. https://doi.org/10.1200/JCO.2005.03.5196
Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S et al (2011) Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA 306(3):269–276. https://doi.org/10.1001/jama.2011.991
Bines J, Oleske DM, Cobleigh MA (1996) Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 14(5):1718–1729. https://doi.org/10.1200/JCO.1996.14.5.1718
Burstein HJ, Winer EP (2000) Primary care for survivors of breast cancer. N Engl J Med 343(15):1086–1094. https://doi.org/10.1056/NEJM200010123431506
Kil WJ, Ahn SD, Shin SS, Lee SW, Choi EK, Kim JH et al (2006) Treatment-induced menstrual changes in very young (%3c35 years old) breast cancer patients. Breast Cancer Res Treat 96(3):245–250. https://doi.org/10.1007/s10549-005-9059-x
Venturini M, Del Mastro L, Aitini E, Baldini E, Caroti C, Contu A et al (2005) Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst 97(23):1724–1733. https://doi.org/10.1093/jnci/dji398
Parulekar WR, Day AG, Ottaway JA, Shepherd LE, Trudeau ME, Bramwell V et al (2005) Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: analysis of a National Cancer Institute of Canada Clinical Trials Group Study–NCIC CTG MA.5. J Clin Oncol 23(25):6002–6008. https://doi.org/10.1200/JCO.2005.07.096
Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352(22):2302–2313. https://doi.org/10.1056/NEJMoa043681
Tham YL, Sexton K, Weiss H, Elledge R, Friedman LC, Kramer R (2007) The rates of chemotherapy-induced amenorrhea in patients treated with adjuvant doxorubicin and cyclophosphamide followed by a taxane. Am J Clin Oncol 30(2):126–132. https://doi.org/10.1097/01.coc.0000251398.57630.4f
Berliere M, Dalenc F, Malingret N, Vindevogel A, Piette P, Roche H et al (2008) Incidence of reversible amenorrhea in women with breast cancer undergoing adjuvant anthracycline-based chemotherapy with or without docetaxel. BMC Cancer 8:56. https://doi.org/10.1186/1471-2407-8-56
Okanami Y, Ito Y, Watanabe C, Iijima K, Iwase T, Tokudome N et al (2011) Incidence of chemotherapy-induced amenorrhea in premenopausal patients with breast cancer following adjuvant anthracycline and taxane. Breast Cancer 18(3):182–188. https://doi.org/10.1007/s12282-011-0256-7
Munhoz RR, Pereira AA, Sasse AD, Hoff PM, Traina TA, Hudis CA et al (2016) Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA oncology 2(1):65–73. https://doi.org/10.1001/jamaoncol.2015.3251
Del Mastro L, Venturini M, Sertoli MR, Rosso R (1997) Amenorrhea induced by adjuvant chemotherapy in early breast cancer patients: prognostic role and clinical implications. Breast Cancer Res Treat 43(2):183–190
Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L et al (2010) Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 362(22):2053–2065. https://doi.org/10.1056/NEJMoa0909638
Vanhuyse M, Fournier C, Bonneterre J (2005) Chemotherapy-induced amenorrhea: influence on disease-free survival and overall survival in receptor-positive premenopausal early breast cancer patients. Ann Oncol 16(8):1283–1288. https://doi.org/10.1093/annonc/mdi241
Swain SM, Jeong JH, Wolmark N (2010) Amenorrhea from breast cancer therapy–not a matter of dose. N Engl J Med 363(23):2268–2270. https://doi.org/10.1056/NEJMc1009616
Giobbie-Hurder A, Gelber RD, Regan MM (2013) Challenges of guarantee-time bias. J Clin Oncol 31(23):2963–2969. https://doi.org/10.1200/JCO.2013.49.5283
Watanabe T, Kuranami M, Inoue K, Masuda N, Aogi K, Ohno S et al (2017) Comparison of an AC-taxane versus AC-free regimen and paclitaxel versus docetaxel in patients with lymph node-positive breast cancer: final results of the National Surgical Adjuvant Study of Breast Cancer 02 trial, a randomized comparative phase 3 study. Cancer 123(5):759–768. https://doi.org/10.1002/cncr.30421
Bycott P, Taylor J (1998) A comparison of smoothing techniques for CD4 data measured with error in a time-dependent Cox proportional hazards model. Stat Med 17(18):2061–2077. https://doi.org/10.1002/(sici)1097-0258(19980930)17:18%3c2061:aid-sim896%3e3.0.co;2-o
Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S et al (2003) Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21(6):976–983. https://doi.org/10.1200/JCO.2003.02.063
Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B et al (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23(16):3686–3696. https://doi.org/10.1200/JCO.2005.10.517
Zavos A, Valachis A (2016) Risk of chemotherapy-induced amenorrhea in patients with breast cancer: a systematic review and meta-analysis. Acta Oncol 55(6):664–670. https://doi.org/10.3109/0284186X.2016.1155738
Ruddy KJ, Guo H, Barry W, Dang CT, Yardley DA, Moy B et al (2015) Chemotherapy-related amenorrhea after adjuvant paclitaxel-trastuzumab (APT trial). Breast Cancer Res Treat 151(3):589–596. https://doi.org/10.1007/s10549-015-3426-z
Walshe JM, Denduluri N, Swain SM (2006) Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 24(36):5769–5779. https://doi.org/10.1200/JCO.2006.07.2793
Han HS, Ro J, Lee KS, Nam BH, Seo JA, Lee DH et al (2009) Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat 115(2):335–342. https://doi.org/10.1007/s10549-008-0071-9
Zhao J, Liu J, Chen K, Li S, Wang Y, Yang Y et al (2014) What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat 145(1):113–128. https://doi.org/10.1007/s10549-014-2914-x
Cho IS, Chae YR, Kim JH, Yoo HR, Jang SY, Kim GR et al (2017) Statistical methods for elimination of guarantee-time bias in cohort studies: a simulation study. BMC Med Res Methodol 17(1):126. https://doi.org/10.1186/s12874-017-0405-6
Acknowledgements
We would like to thank the patients, investigators, and institutions involved in this study. We would also like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was funded by the Comprehensive Support Project for Oncology Research (CSPOR) of Public Health Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FH, YU and HM. The first draft of the manuscript was written by TI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
FH received personal fees from Chugai, Kyowa Kirin, Eisai, Pfizer, and Eli Lilly, outside the submitted work. HM received honoraria from AstraZeneca, Pfizer, Takeda, Daiichi Sankyo and Taiho, and research grant from Japanese government, Daiichi Sankyo, Eisai, Nippon Kayaku and Pfizer, outside the submitted work. TW received advisory fee from CHUGAI PHARMACEUTICAL CO., LTD., TAIHO PHARMACEUTICAL CO., LTD., outside the submitted work. YO received personal fees from Statcom, Daiichi Sankyo, Chugai, Shionogi, Taiho, Sanofi and EP-Crsu and grants from Medical Member System, outside the submitted work. TI and YU declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board at National Hospital Organization Shikoku Cancer Center and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent Informed consent was not required for this retrospective analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1—The Kaplan-Maier estimates of DFS for CIA and non-CIA groups. DFS was evaluated separately for (a) all, (b) ER-positive, and (c) ER-negative patients. DFS: Disease-free survival; CIA: chemotherapy induced amenorrhea; ER: Estrogen receptor. Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iwamoto, T., Hara, F., Uemura, Y. et al. NSAS-BC02 substudy of chemotherapy-induced amenorrhea (CIA) in premenopausal patients who received either taxane alone or doxorubicin(A) cyclophosphamide(C) followed by taxane as postoperative chemotherapy. Breast Cancer Res Treat 182, 325–332 (2020). https://doi.org/10.1007/s10549-020-05692-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05692-5