Abstract
Purpose
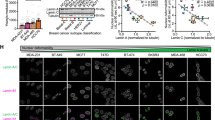
Lamins A/C, a major component of the nuclear lamina, play key roles in maintaining nuclear integrity, regulation of gene expression, cell proliferation and apoptosis. Reduced lamin A/C expression in cancer has been reported to be a sign of poor prognosis. However, its clinical significance in breast cancer remains to be defined. This study aimed to evaluate expression and prognostic significance of lamin A/C in early-stage breast cancer.
Methods
Using immunohistochemical staining of tissue microarrays, expression of lamin A/C was evaluated in a large well-characterised series of early-stage operable breast cancer (n = 938) obtained from Nottingham Primary Breast Carcinoma Series. Association of lamin A/C expression with clinicopathological parameters and outcome was evaluated.
Results
Positive expression rate of lamin A/C in breast cancer was 42.2% (n = 398). Reduced/loss of expression of lamin A/C was significantly associated with high histological grade (p < 0.001), larger tumour size (p = 0.004), poor Nottingham Prognostic Index score (p < 0.001), lymphovascular invasion (p = 0.014) and development of distant metastasis (p = 0.027). Survival analysis showed that reduced/loss of expression of lamin A/C was significantly associated with shorter breast cancer-specific survival (p = 0.008).
Conclusion
This study suggests lamin A/C plays a role in breast cancer and loss of its expression is associated with variables of poor prognosis and shorter outcome.




Similar content being viewed by others
References
Lammerding J, Fong LG, Ji JY et al (2006) Lamins a and C but not lamin B1 regulate nuclear mechanics. J Biol Chem 281:25768–25780. https://doi.org/10.1074/jbc.M513511200
Butin-Israeli V, Adam SA, Goldman AE, Goldman RD (2012) Nuclear lamin functions and disease. Trends Genet 28:464–471. https://doi.org/10.1016/j.tig.2012.06.001
Kong L, Schäfer G, Bu H et al (2012) Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway. Carcinogenesis 33:751–759. https://doi.org/10.1093/carcin/bgs022
Eckersley-Maslin MA, Bergmann JH, Lazar Z, Spector DL (2013) Lamin A/C is expressed in pluripotent mouse embryonic stem cells. Nucleus 4:53–60. https://doi.org/10.4161/nucl.23384
Marmiroli S, Bertacchini J, Beretti F et al (2009) A-type lamins and signaling: the PI 3-kinase/Akt pathway moves forward. J Cell Physiol 220:553–561. https://doi.org/10.1002/jcp.21807
Jung H, Lee J, Yang S et al (2013) Nuclear lamins in the brain—new insights into function and regulation. Mol Neurobiol 47:290–301. https://doi.org/10.1007/s12035-012-8350-1.Nuclear
Gerace L, Comeau C, Benson M (1984) Organization and modulation of nuclear lamina structure. J Cell Sci Suppl 1:137–160
Ivorra C, Kubicek M, González JM et al (2006) A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev 20:307–320. https://doi.org/10.1101/gad.349506
Houben F, Willems CHMP, Declercq ILJ et al (2009) Disturbed nuclear orientation and cellular migration in A-type lamin deficient cells. Biochim Biophys Acta Mol Cell Res 1793:312–324. https://doi.org/10.1016/J.BBAMCR.2008.10.003
Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J (2013) Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497:507–513. https://doi.org/10.1038/nature12105
Lee KK, Haraguchi T, Lee RS et al (2001) Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J Cell Sci 114:4567–4573
Worman HJ (2012) Nuclear lamins and laminopathies. J Pathol 226:316–325. https://doi.org/10.1002/path.2999
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Zink D, Fische AH, Nickerson JA (2004) Nuclear structure in cancer cells. Nat Rev Cancer 4:677–687. https://doi.org/10.1038/nrc1430
Solovei I, Wang AS, Thanisch K et al (2013) LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152:584–598. https://doi.org/10.1016/j.cell.2013.01.009
Brachner A, Foisner R (2014) Lamina-associated polypeptide (LAP)2α and other LEM proteins in cancer biology. Adv Exp Med Biol 773:143–163. https://doi.org/10.1007/978-1-4899-8032-8_7
Qi Y-X, Yao Q-P, Huang K et al (2016) Nuclear envelope proteins modulate proliferation of vascular smooth muscle cells during cyclic stretch application. Proc Natl Acad Sci USA 113:5293–5298. https://doi.org/10.1073/pnas.1604569113
Markiewicz E, Dechat T, Foisner R et al (2002) Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell 13:4401–4413. https://doi.org/10.1091/mbc.E02-07-0450
Lin F, Worman HJ (1993) Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem 268:16321–16326
Tilli CM, Ramaekers FC, Broers JL et al (2003) Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. Br J Dermatol 148:102–109
Broers JL, Raymond Y, Rot MK et al (1993) Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am J Pathol 143:211–220
Kaspi E, Frankel D, Guinde J et al (2017) Low lamin A expression in lung adenocarcinoma cells from pleural effusions is a pejorative factor associated with high number of metastatic sites and poor performance status. PLoS ONE 12:e0183136. https://doi.org/10.1371/journal.pone.0183136
Moss SF, Krivosheyev V, de Souza A et al (1999) Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut 45:723–729
Prokocimer M, Margalit A, Gruenbaum Y (2006) The nuclear lamina and its proposed roles in tumorigenesis: projection on the hematologic malignancies and future targeted therapy. J Struct Biol 155:351–360. https://doi.org/10.1016/j.jsb.2006.02.016
Wu Z, Wu L, Weng D et al (2009) Reduced expression of lamin A/C correlates with poor histological differentiation and prognosis in primary gastric carcinoma. J Exp Clin Cancer Res 28:8. https://doi.org/10.1186/1756-9966-28-8
Belt EJT, Fijneman RJA, van den Berg EG et al (2011) Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. Eur J Cancer 47:1837–1845. https://doi.org/10.1016/j.ejca.2011.04.025
Wazir U, Ahmed M, Bridger J et al (2013) The clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor mRNA expression in human breast cancer. Cell Mol Biol Lett 18:595–611. https://doi.org/10.2478/s11658-013-0109-9
Matsumoto A, Hieda M, Yokoyama Y et al (2015) Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med 4:1547–1557. https://doi.org/10.1002/cam4.495
Capo-chichi CD, Cai KQ, Smedberg J et al (2011) Loss of A-type lamin expression compromises nuclear envelope integrity in breast cancer. Chin J Cancer 30:415–425
Aljada A, Doria J, Saleh AM et al (2016) Altered Lamin A/C splice variant expression as a possible diagnostic marker in breast cancer. Cell Oncol 39:161–174. https://doi.org/10.1007/s13402-015-0265-1
Abd El-Rehim DM, Ball G, Pinder SE et al (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116:340–350. https://doi.org/10.1002/ijc.21004
Kollias J, Murphy CA, Elston CW et al (1999) The prognosis of small primary breast cancers. Eur J Cancer 35:908–912
Madjd Z, Pinder SE, Paish C et al (2003) Loss of CD59 expression in breast tumours correlates with poor survival. J Pathol 200:633–639. https://doi.org/10.1002/path.1357
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259
Agrelo R, Setien F, Espada J et al (2005) Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. J Clin Oncol 23:3940–3947. https://doi.org/10.1200/JCO.2005.11.650
Jones PA, Laird PW (1999) Cancer epigenetics comes of age. Nat Genet 21:163–167. https://doi.org/10.1038/5947
Baylin SB, Esteller M, Rountree MR et al (2001) Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet 10:687–692
Widschwendter M, Jones PA (2002) DNA methylation and breast carcinogenesis. Oncogene 21:5462–5482. https://doi.org/10.1038/sj.onc.1205606
Yan PS, Venkataramu C, Ibrahim A et al (2006) Mapping geographic zones of cancer risk with epigenetic biomarkers in normal breast tissue. Clin Cancer Res 12:6626–6636. https://doi.org/10.1158/1078-0432.CCR-06-0467
Cheng ASL, Culhane AC, Chan MWY et al (2008) Epithelial progeny of estrogen-exposed breast progenitor cells display a cancer-like methylome. Cancer Res 68:1786–1796. https://doi.org/10.1158/0008-5472.CAN-07-5547
Sunami E, Shinozaki M, Sim MS et al (2008) Estrogen receptor and HER2/neu status affect epigenetic differences of tumor-related genes in primary breast tumors. Breast Cancer Res 10:R46. https://doi.org/10.1186/bcr2098
Jing F, Yuping W, Yong C et al (2010) CpG island methylator phenotype of multigene in serum of sporadic breast carcinoma. Tumour Biol 31:321–331. https://doi.org/10.1007/s13277-010-0040-x
Karray-Chouayekh S, Trifa F, Khabir A et al (2010) Aberrant methylation of RASSF1A is associated with poor survival in Tunisian breast cancer patients. J Cancer Res Clin Oncol 136:203–210. https://doi.org/10.1007/s00432-009-0649-6
Sharma G, Mirza S, Parshad R et al (2010) Clinical significance of promoter hypermethylation of DNA repair genes in tumor and serum DNA in invasive ductal breast carcinoma patients. Life Sci 87:83–91. https://doi.org/10.1016/j.lfs.2010.05.001
Swift-Scanlan T, Vang R, Blackford A et al (2011) Methylated genes in breast cancer: associations with clinical and histopathological features in a familial breast cancer cohort. Cancer Biol Ther 11:853–865
Irianto J, Pfeifer CR, Ivanovska IL et al (2016) Nuclear lamins in cancer. Cell Mol Bioeng 9:258–267. https://doi.org/10.1007/s12195-016-0437-8
Ferguson AT, Lapidus RG, Baylin SB, Davidson NE (1995) Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res 55:2279–2283
Momparler RL, Bovenzi V (2000) DNA methylation and cancer. J Cell Physiol 183:145–154. https://onlinelibrary.wiley.com/doi/abs/10.1002/%28SICI%291097-4652%28200005%29183%3A2%3C145%3A%3AAID-JCP1%3E3.0.CO%3B2-V
Tommasi S, Karm DL, Wu X et al (2009) Methylation of homeobox genes is a frequent and early epigenetic event in breast cancer. Breast Cancer Res 11:R14. https://doi.org/10.1186/bcr2233
Yang L, Guan T, Gerace L (1997) Lamin-binding fragment of LAP2 inhibits increase in nuclear volume during the cell cycle and progression into S phase. J Cell Biol 139:1077–1087
Acknowledgements
We thank the Nottingham Health Science Biobank and Breast Cancer Now Tissue Bank for the provision of tissue samples. The authors gratefully acknowledge the support of the Libyan Authority for Research, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Nottingham Research Ethics Committee 2 under the title “Development of a molecular genetic classification of breast cancer”.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Alhudiri, I.M., Nolan, C.C., Ellis, I.O. et al. Expression of Lamin A/C in early-stage breast cancer and its prognostic value. Breast Cancer Res Treat 174, 661–668 (2019). https://doi.org/10.1007/s10549-018-05092-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-05092-w