Abstract
Purpose
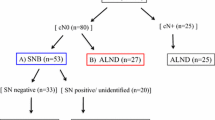
Many breast cancer patients with positive axillary lymph nodes achieve complete node remission after neoadjuvant chemotherapy. The usefulness of sentinel lymph node biopsy in this situation is uncertain. This study evaluated the outcomes of sentinel biopsy-guided decisions in patients who had conversion of axillary nodes from clinically positive to negative following neoadjuvant chemotherapy.
Methods
We reviewed the records of 1247 patients from five hospitals in Korea who had breast cancer with clinically axillary lymph node-positive status and negative conversion after neoadjuvant chemotherapy, between 2005 and 2012. Patients who underwent axillary operations with sentinel biopsy-guided decisions (Group A) were compared with patients who underwent complete axillary lymph node dissection without sentinel lymph node biopsy (Group B). Axillary node recurrence and distant recurrence-free survival were compared.
Results
There were 428 cases in Group A and 819 in Group B. Kaplan–Meier analysis showed that recurrence-free survivals were not significantly different between Groups A and B (4-year axillary recurrence-free survival: 97.8 vs. 99.0%; p = 0.148). Multivariate analysis also indicated the two groups had no significant difference in axillary and distant recurrence-free survival.
Conclusions
For breast cancer patients who had clinical conversion of axillary lymph nodes from positive to negative following neoadjuvant chemotherapy, sentinel biopsy-guided axillary surgery, and axillary lymph node dissection without sentinel lymph node biopsy had similar rates of recurrence. Thus, sentinel biopsy-guided axillary operation in breast cancer patients who have clinically axillary lymph node positive to negative conversion following neoadjuvant chemotherapy is a useful strategy.



Similar content being viewed by others
References
Schwartz GF, Giuliano AE, Veronesi U, Consensus Conference C (2002) Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer 94(10):2542–2551
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Mammolito DM, McCready DR, Mamounas EP, Costantino JP, Wolmark N, National Surgical Adjuvant B, Bowel P (2007) Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 8(10):881–888. doi:10.1016/S1470-2045(07)70278-4
Kuerer HM, Sahin AA, Hunt KK, Newman LA, Breslin TM, Ames FC, Ross MI, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg 230(1):72–78
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horvath Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M, Neo AST (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379(9816):633–640. doi:10.1016/S0140-6736(11)61847-3
King TA, Morrow M (2015) Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol 12(6):335–343. doi:10.1038/nrclinonc.2015.63
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Kuerer HM, Bowling M, Flippo-Morton TS, Byrd DR, Ollila DW, Julian TB, McLaughlin SA, McCall L, Symmans WF, Le-Petross HT, Haffty BG, Buchholz TA, Nelson H, Hunt KK, Alliance for Clinical Trials in O (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310(14):1455–1461. doi:10.1001/jama.2013.278932
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, Liedtke C, von Minckwitz G, Nekljudova V, Schmatloch S, Schrenk P, Staebler A, Untch M (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14(7):609–618. doi:10.1016/S1470-2045(13)70166-9
Michel SC, Keller TM, Frohlich JM, Fink D, Caduff R, Seifert B, Marincek B, Kubik-Huch RA (2002) Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology 225(2):527–536. doi:10.1148/radiol.2252011605
Mortellaro VE, Marshall J, Singer L, Hochwald SN, Chang M, Copeland EM, Grobmyer SR (2009) Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging 30(2):309–312. doi:10.1002/jmri.21802
Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, Holmes DR, Sposto R, Sener SF (2012) Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol 19(6):1825–1830. doi:10.1245/s10434-011-2200-7
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN (2006) Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg 93(5):539–546. doi:10.1002/bjs.5209
Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, Arnaout A, Brackstone M, McCready DR, Karp SE, Trop I, Lisbona A, Wright FC, Younan RJ, Provencher L, Patocskai E, Omeroglu A, Robidoux A (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 33(3):258–264. doi:10.1200/JCO.2014.55.7827
Mittendorf EA, Caudle AS, Yang W, Krishnamurthy S, Shaitelman S, Chavez-MacGregor M, Woodward WA, Bedrosian I, Kuerer HM, Hunt KK (2014) Implementation of the american college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol 21(8):2468–2473. doi:10.1245/s10434-014-3775-6
Galimberti V, Ribeiro Fontana SK, Maisonneuve P, Steccanella F, Vento AR, Intra M, Naninato P, Caldarella P, Iorfida M, Colleoni M, Viale G, Grana CM, Rotmensz N, Luini A (2016) Sentinel node biopsy after neoadjuvant treatment in breast cancer: five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol 42(3):361–368. doi:10.1016/j.ejso.2015.11.019
Kim JY, Kim MK, Lee JE, Jung Y, Bae SY, Lee SK, Kil WH, Kim SW, Kim KS, Nam SJ, Han S (2015) Sentinel lymph node biopsy alone after neoadjuvant chemotherapy in patients with initial cytology-proven axillary node metastasis. J Breast Cancer 18(1):22–28. doi:10.4048/jbc.2015.18.1.22
Han A, Moon HG, Kim J, Ahn SK, Park IA, Han W, Noh DY (2013) Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 16(4):378–385. doi:10.4048/jbc.2013.16.4.378
Kang SH, Kang JH, Choi EA, Lee ES (2004) Sentinel lymph node biopsy after neoadjuvant chemotherapy. Breast Cancer 11(3):233–241 (Discussion 264–236)
Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N (2002) Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. New Engl J Med 347(8):567–575. doi:10.1056/NEJMoa020128
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Wolmark N (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11(10):927–933. doi:10.1016/S1470-2045(10)70207-2
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K (2010) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 252(3):426–432. doi:10.1097/SLA.0b013e3181f08f32
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U, International Breast Cancer Study Group Trial I (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 14(4):297–305. doi:10.1016/S1470-2045(13)70035-4
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JH, Orzalesi L, Bouma WH, van der Mijle HC, Nieuwenhuijzen GA, Veltkamp SC, Slaets L, Duez NJ, de Graaf PW, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JW, Belkacemi Y, Petignat P, Schinagl DA, Coens C, Messina CG, Bogaerts J, Rutgers EJ (2014) Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 15(12):1303–1310. doi:10.1016/S1470-2045(14)70460-7
Gentilini O, Veronesi U (2012) Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: sentinel node vs observation after axillary UltraSouND). Breast 21(5):678–681. doi:10.1016/j.breast.2012.06.013
Reimer T, Hartmann S, Stachs A, Gerber B (2014) Local treatment of the axilla in early breast cancer: concepts from the national surgical adjuvant breast and bowel project B-04 to the planned intergroup sentinel mamma trial. Breast Care (Basel) 9(2):87–95. doi:10.1159/000360411
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Kang, YJ., Han, W., Park, S. et al. Outcome following sentinel lymph node biopsy-guided decisions in breast cancer patients with conversion from positive to negative axillary lymph nodes after neoadjuvant chemotherapy. Breast Cancer Res Treat 166, 473–480 (2017). https://doi.org/10.1007/s10549-017-4423-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4423-1