Abstract
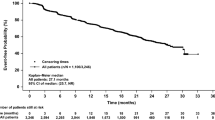
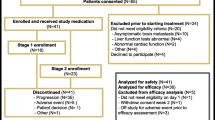
In murine models, overexpression of the MET receptor transgene induces tumors with human basal gene expression characteristics supporting MET inhibition as a treatment strategy for triple-negative breast cancer (TNBC). Foretinib is an oral multi-kinase inhibitor of MET, RON, AXL, TIE-2, and VEGF receptors with anti-tumor activity in advanced HCC and papillary renal cell cancer. Patients with centrally reviewed primary TNBC and 0–1 prior regimens for metastatic disease received daily foretinib 60 mg po in a 2-stage single-arm trial. Primary endpoints were objective response and early progression rates per RECIST 1.1. In stage 2, correlative studies of MET, PTEN, EGFR, and p53 on archival and fresh tumor specimens were performed along with enumeration of CTCs. 45 patients were enrolled with 37 patients having response evaluable and centrally confirmed primary TNBC (cTNBC). There were 2 partial responses (ITT 4.7 % response evaluable cTNBC 5.4 %) with a median duration of 4.4 months (range 3.7–5 m) and 15 patients had stable disease (ITT 33 %, response evaluable cTNBC 40.5 %) with a median duration of 5.4 months (range 2.3–9.7 m). The most common toxicities (all grades/grade 3) were nausea (64/4 %), fatigue (60/4 %), hypertension (58/49 %), and diarrhea (40/7 %). Six serious adverse events were considered possibly related to foretinib and 4 patients went off study due to adverse events. There was no correlation between MET positivity and response nor between response and PTEN, EGFR, p53, or MET expression in CTCs. Although CCTG IND 197 did not meet its primary endpoint, the observation of a clinical benefit rate of 46 % in this cTNBC population suggests that foretinib may have clinical activity as a single, non-cytotoxic agent in TNBC (ClinicalTrials.gov number, NCT01147484).


Similar content being viewed by others
References
Rakha EA, Reis-Filho JS, Ellis IO (2008) Basal-like breast cancer: a critical review. J Clin Oncol 26:2568–2581
Metzger-Filho O, Tutt, de Azambuja E et al (2012) Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol 30:1879–1887
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Appleman LJ (2011) MET signaling pathway: a rational target for cancer therapy. J Clin Oncol 29:4837–4838
Maroun CR, Rowlands T (2014) The MET receptor tyrosine kinase: a key player in oncogenesis and drug resistance. Pharmacol Ther 142:316–338
Blumenschein GR, Mills GB, Gonzalez-Angulo AM (2012) Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol 30:3287–3296
Ho-Yen CM, Green AR, Rakha EA et al (2013) C-met in invasive breast cancer. Cancer 120:163–171
Qian F, Engst S, Yamaguchi K et al (2009) Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res 69:8009–8016
Zhu K, Kong X, Zhao D et al (2014) C-MET kinase inhibitors: a patent review (2011–2013). Expert Opin Ther Pat 24:217–230
Dent S, Zee B, Dancey J et al (2001) Application of a new multinomial phase II stopping rule using response and early progression. J Clin Oncol 19:785–791
Freidlin B, Dancey J, Korn EL (2002) Multinomial phase II designs. J Clin Oncol 20:599
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Capuzzo F, Hirsch FR, Rossi E et al (2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. H Natl Cancer Inst 97(9):643–655
Mayer IA, Abramson VG, Lehmann BD et al (2014) New strategies for triple negative breast cancer-deciphering the heterogeneity. Clin Cancer Res 20:782–790
Jardim DL, Tang C, Gagliato DDM et al (2014) Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson phase I clinic. Clin Cancer Res 20:6336–6345
Gelmon K, Dent R, Mackey JR et al (2012) Targeting triple-negative breast cancer: optimizing therapeutic outcomes. Ann Oncol 23:2223–2234
Tutt A, Ellis P, Kilburn L et al (2014) TNT: a randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA ½ breast cancer (CRUK/07/012). 2014 San Antonio Breast Cancer Symposium, San Antonio TX, USA. Publication number S3-01, Dec 9–13
Bramati A, Girelli S, Torri V et al (2014) Efficacy of biological agents in metastatic triple-negative breast cancer. Cancer Treat Rev 40:605–613
Gaule PB, Crown J, O’Donovan N et al (2014) CMET in triple-negative breast cancer: is it a therapeutic target for this subset of breast cancer patients? Expert Opin Ther Targets 18:999–1009
Acknowledgements
Canadian Cancer Trials Group was supported by the Canadian Cancer Society Research Institute to the Canadian Cancer Trials Group (grant #021039).
Funding
PB was funded by a Cancer Care Ontario Research Chair in Experimental Therapeutics. Fellow (PB) was funded by AstraZeneca—CCTG Drug Development Fellowship and the Terry Fox Foundation Training Program in Transdisciplinary Cancer Research in partnership with CIHR, Canadian Cancer Trials Group Development Fellowship
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Potential conflict of interest statements
Dr. Rayson has received honoraria from Ipsen, Amgen, and Novartis and has served as a consultant for Ipsen and Novartis. Dr Lupichuk has served as a consultant for Genomic Health. Dr Potvin has received honoraria from Novartis, Pfizer, and Janssen and has been on a speakers’ bureau for Novartis and Pfizer. Dr Dent has received honoraria from Roche, Amgen, and Astra Zeneca and has served as a consultant for Novartis. Dr Shenkier has received honoraria from Novartis and Roche and has served as a consultant for Novartis, Roche, and Janssen. Dr Ellard owns stock in Pfizer, Abbvie, and GSK and has served as a consultant for GSK. Dr Prady has served as a consultant for AstraZeneca. Dr Allo reports spousal employment with GSK. Dr Goodwin has served as a consultant for Celgene, Novartis, BMS, Amgen, and Ipsen. Drs Dhesy-Thind, Salim, Farmer, Tsao, Allan, Ludkovski, Bonomi, Tu, Eisenhauer, and Bradbury report no potential conflicts of interest as does Ms. Hagerman. GSK provided foretinib and supplied funding to the Canadian Cancer Trials Group to support IND 197 but was not involved with the trial design, data analysis, or reporting of results.
Rights and permissions
About this article
Cite this article
Rayson, D., Lupichuk, S., Potvin, K. et al. Canadian Cancer Trials Group IND197: a phase II study of foretinib in patients with estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2-negative recurrent or metastatic breast cancer. Breast Cancer Res Treat 157, 109–116 (2016). https://doi.org/10.1007/s10549-016-3812-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3812-1