Abstract
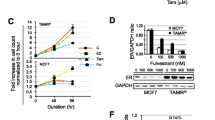
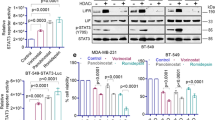
Several publications have suggested that histone deacetylase inhibitors (HDACis) could reverse the repression of estrogen receptor alpha (ERα) in triple-negative breast cancer (TNBC) cell lines, leading to the induction of a functional protein. Using different HDACis, vorinostat, panobinostat, and abexinostat, we therefore investigated this hypothesis in various human TNBC cell lines and patient-derived xenografts (PDXs). We used three human TNBC cell lines and three PDXs. We analyzed the in vitro toxicity of the compounds, their effects on the hormone receptors and hormone-related genes and protein expression both in vitro and in vivo models. We then explored intra-tumor histone H3 acetylation under abexinostat in xenograft models. Despite major cytotoxicity of all tested HDAC inhibitors and repression of deactylation-dependent CCND1 gene, neither ERα nor ERβ, ESR1 or ESR2 genes respectively, were re-expressed in vitro. In vivo, after administration of abexinostat for three consecutive days, we did not observe any induction of ESR1 or ESR1-related genes and ERα protein expression by RT-qPCR and immunohistochemical methods in PDXs. This observation was concomitant to the fact that in vivo administration of abexinostat increased intra-tumor histone H3 acetylation. These observations do not allow us to confirm previous studies which suggested that HDACis are able to convert ER-negative (ER−) tumors to ER-positive (ER+) tumors, and that a combination of HDAC inhibitors and hormone therapy could be proposed in the management of TNBC patients.




Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62(1):10–29
Cuzick J, Sestak I, Baum M et al (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11(12):1135–1141
Linares A, Dalenc F, Balaguer P et al (2011) Manipulating protein acetylation in breast cancer: a promising approach in combination with hormonal therapies? J Biomed Biotechnol. doi:10.1155/2011/856985
Li Y, Meeran SM, Patel SN, Chen H, Hardy TM, Tollefsbol TO (2013) Epigeneticreactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol Cancer 12:9
Zhou Q, Shaw PG, Davidson NE (2009) Inhibition of histone deacetylase suppresses EGFsignaling pathways by destabilizing EGFR mRNA in ER-negative human breast cancer cells. Breast Cancer Res Treat 117(2):443–451
Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE (2005) Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol 19(7):1740–1751
Zhou Q, Atadja P, Davidson NE (2007) Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol Ther 6(1):64–69
Leu YW, Yan PS, Fan M, Jin VX, Liu JC, Curran EM, Welshons WV, Wei SH, Davuluri RV, Plass C, Nephew KP, Huang TH (2004) Loss of estrogen receptor signaling triggers epigenetic silencing of downstream targets in breast cancer. Cancer Res 64(22):8184–8192
Sabnis GJ, Goloubeva O, Chumsri S et al (2011) Functional activation of the estrogen receptor-alpha and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res 71(5):1893–1903
Sharma D, Saxena NK, Davidson NE, Vertino PM (2006) Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res 66(12):6370–6378
Grant S, Dai Y (2012) Histone deacetylase inhibitors and rational combination therapies. Adv Cancer Res 116:199–237
Ververis K, Hiong A, Karagiannis TC, Licciardi PV (2013) Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics 7:47–60
Bose P, Dai Y, Grant S (2014) Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol Ther 143(3):323–336
Marangoni E, Vincent-Salomon A, Auger N et al (2007) A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 13(13):3989–3998
Reyal F, Guyader C, Decraene C et al (2012) Molecular profiling of patient-derived breast cancer xenografts. Breast Cancer Res 14(1):R11
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767
Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA, Committee of the National Cancer ResearchInstitute (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102(11):1555–1577
de Cremoux P, Bieche I, Tran-Perennou C et al (2004) Inter-laboratory quality control for hormone-dependent gene expression in human breast tumors using real-time reverse transcription-polymerase chain reaction. Endocr Relat Cancer 11(3):489–495
Lehmann-Che J, Hamy AS, Porcher R, Barritault M, Bouhidel F, Habuellelah H, Leman-Detours S, de Roquancourt A, Cahen-Doidy L, Bourstyn E, de Cremoux P, de Bazelaire C, Albiter M, Giacchetti S, Cuvier C, Janin A, Espié M, de Thé H, Bertheau P (2013) Molecular apocrine breast cancers are aggressive estrogen receptor negative tumors overexpressing either HER2 or GCDFP15. Breast Cancer Res 15(3):R37
Lehmann-Che J, Amira-Bouhidel F, Turpin E, Antoine M, Soliman H, Legres L, Bocquet C, Bernoud R, Flandre E, Varna M, de Roquancourt A, Plassa LF, Giacchetti S, Espié M, de Bazelaire C, Cahen-Doidy L, Bourstyn E, Janin A, de Thé H, Bertheau P (2011) Immunohistochemical and molecular analyses of HER2 status in breast cancers are highly concordant and complementary approaches. Br J Cancer 104(11):1739–1746
de Cremoux P, Rosenberg D, Goussard J et al (2008) Expression of progesterone and estradiol receptors in normal adrenal cortex, adrenocortical tumors, and primary pigmented nodular adrenocortical disease. Endocr Relat Cancer 15(2):465–474
Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE (2001) Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res 61(19):7025–7029
Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ (2007) Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat 105(3):297–309
Biçaku E, Marchion DC, Schmitt ML, Münster PN (2008) Selective inhibition of histone deacetylase 2 silences progesterone receptor-mediated signaling. Cancer Res 68(5):1513–1519
Fleury L, Gerus M, Lavigne AC, Richard-Foy H, Bystricky K (2008) Eliminating epigenetic barriers induces transient hormone-regulated gene expression in estrogen receptor negative breast cancer cells. Oncogene 27(29):4075–4085
Sabnis GJ, Goloubeva OG, Kazi AA, Shah P, Brodie AH (2013) HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2. Mol Cancer Ther 12(12):2804–2816
Rhodes LV, Tate CR, Segar HC, Burks HE, Phamduy TB, Hoang V, Elliott S, Gilliam D, Pounder FN, Anbalagan M, Chrisey DB, Rowan BG, Burow ME, Collins-Burow BM (2014) Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators. Breast Cancer Res Treat 145(3):593–604
Shah P, Gau Y, Sabnis G (2014) Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res Treat 143(1):99–111
Acknowledgments
We thank Dr V Cavaillès, and Mrs C Brunin, E Wittmer, J Wang, A Pontisso, A Chomel, D Meseure, and C Laurent for their helpful contribution to this work. The study was supported by Servier Laboratories. However, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Various co-authors, i.e., Gaëlle Rolland, Laurence Kraus-Berthier, Brian Paul Lockhar, and Stéphane Depil, are employees of Servier Laboratories which is developing abexinostat clinical therapeutic approach. The remaining authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Cremoux, P., Dalvai, M., N’Doye, O. et al. HDAC inhibition does not induce estrogen receptor in human triple-negative breast cancer cell lines and patient-derived xenografts. Breast Cancer Res Treat 149, 81–89 (2015). https://doi.org/10.1007/s10549-014-3233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3233-y