Abstract
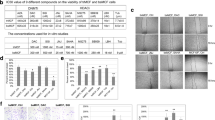
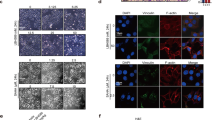
Loss of ERα in breast cancer correlates with poor prognosis, increased recurrence rates, and higher incidence of metastasis. Epigenetic silencing of E-cadherin (loss of which is associated with more invasive phenotype) is observed in metastatic cell lines and invasive breast cancers. Here, we are showing that entinostat (ENT) can reverse epithelial to mesenchymal transition (EMT), which is considered to be a first step in the process of metastases formation. Triple-negative breast cancer cells such as MDA-MB-231 and Hs578T show a basal phenotype characterized by loss of E-cadherin expression and higher expression of mesenchymal markers such as N-cadherin and vimentin along with transcriptional repressors such as twist and snail. When MDA-MB-231 and Hs578T cells or tumors were treated with ENT, E-cadherin transcription was increased along with reduction in N-cadherin mRNA expression. Chromatin immunoprecipitation assay showed that treatment of MDA-MB-231 and Hs578T cells increased the activation of E-cadherin promoter by reducing the association of twist and snail with the E-cadherin (CDH1) promoter and downregulated both the snail and twist. ENT also inhibited cell migration in vitro. In addition, phosphorylation of vimentin was increased, as well as remodeling of vimentin filaments. ENT treatment also reduced formation of tubulin-based microtentacles, which help floating cells attach to other surfaces. These findings suggest that ENT can reverse EMT and may reduce the formation of metastasis.







Similar content being viewed by others
Abbreviations
- ER:
-
Estrogen receptor
- AIs:
-
Aromatase inhibitors
- HDACi:
-
Histone deacetylase inhibitors
- Let:
-
Letrozole
- ENT:
-
Entinostat
References
Sabnis GJ, Goloubeva O, Chumsri S, Nguyen N, Sukumar S, Brodie AM (2011) Functional activation of the estrogen receptor-alpha; and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res 71:1893–1903
Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW (2008) Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis 25:629–642. doi:10.1007/s10585-008-9170-6
Cowin P, Welch DR (2007) Breast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia 12:99–102. doi:10.1007/s10911-007-9041-9
Tse JC, Kalluri R (2007) Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem 101:816–829. doi:10.1002/jcb.21215
Fearon ER (2003) Connecting estrogen receptor function, transcriptional repression, and E-cadherin expression in breast cancer. Cancer Cell 3:307–310
Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, Cleton-Jansen AM (2006) E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer 94:661–671
Tamura G, Yin J, Wang S, Fleisher AS, Zou T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, Silverberg SG, Nishizuka S, Terashima M, Motoyama T, Meltzer SJ (2000) E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 92:569–573
van Horssen R, Hollestelle A, Rens JA, Eggermont AM, Schutte M, Ten Hagen TL (2012) E-cadherin promotor methylation and mutation are inversely related to motility capacity of breast cancer cells. Breast Cancer Res Treat 136:365–377. doi:10.1007/s10549-012-2261-8
Zou D, Yoon HS, Perez D, Weeks RJ, Guilford P, Humar B (2009) Epigenetic silencing in non-neoplastic epithelia identifies E-cadherin (CDH1) as a target for chemoprevention of lobular neoplasia. J Pathol 218:265–272. doi:10.1002/path.2541
Peinado H, Ballestar E, Esteller M, Cano A (2004) Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24:306–319
Vesuna F, van Diest P, Chen JH, Raman V (2008) Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun 367:235–241. doi:10.1016/j.bbrc.2007.11.151
Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, Yoon JR, Ioffe OB, Tuttle KC, Tan M, Martin SS (2010) Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene 29:3217–3227. doi:10.1038/onc.2010.68
Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS (2008) Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res 68:5678–5688. doi:10.1158/0008-5472.CAN-07-6589
Whipple RA, Matrone MA, Cho EH, Balzer EM, Vitolo MI, Yoon JR, Ioffe OB, Tuttle KC, Yang J, Martin SS (2010) Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res 70:8127–8137. doi:10.1158/0008-5472.CAN-09-4613
Sabnis GJ, Jelovac D, Long B, Brodie A (2005) The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res 65:3903–3910
Solly K, Wang X, Xu X, Strulovici B, Zheng W (2004) Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol 2:363–372. doi:10.1089/1540658041850544
Ungefroren H, Sebens S, Groth S, Gieseler F, Fandrich F (2011) Differential roles of Src in transforming growth factor-β regulation of growth arrest, epithelial-to-mesenchymal transition and cell migration in pancreatic ductal adenocarcinoma cells. Int J Oncol 38:797–805. doi:10.3892/ijo.2011.897
Whipple RA, Vitolo MI, Boggs AE, Charpentier MS, Thompson K, Martin SS (2013) Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-kappaB inhibition. Breast Cancer Res 15:R83. doi:10.1186/bcr3477
Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A (2009) Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res 69:1416–1428
Schech AJ, Kazi AA, Gilani RA, Brodie AH (2013) Zoledronic acid reverses the epithelial-mesenchymal Transition and Inhibits Self-Renewal of Breast Cancer Cells through Inactivation of NF-kappaB. Mol Cancer Ther. doi:10.1158/1535-7163.MCT-12-0304
Kazi AA, Jones JM, Koos RD (2005) Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor alpha and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol 19:2006–2019
Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD (2004) Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci 117:919–932. doi:10.1242/jcs.00906
Satelli A, Li S (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cellular and molecular life sciences. CMLS 68:3033–3046. doi:10.1007/s00018-011-0735-1
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502. doi:10.1001/jama.295.21.2492
Curigliano G, Goldhirsch A (2011) The triple-negative subtype: new ideas for the poorest prognosis breast cancer. J Natl Cancer Inst Monogr 2011:108–110. doi:10.1093/jncimonographs/lgr038
Brady-West DC, McGrowder DA (2011) Triple negative breast cancer: therapeutic and prognostic implications. APJCP 12:2139–2143
Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39:305–318. doi:10.1080/00313020701329914
Guarino M (2007) Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol 39:2153–2160. doi:10.1016/j.biocel.2007.07.011
van Roy F, Berx G (2008) The cell–cell adhesion molecule E-cadherin. CMLS 65:3756–3788. doi:10.1007/s00018-008-8281-1
Baranwal S, Alahari SK (2009) Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun 384:6–11. doi:10.1016/j.bbrc.2009.04.051
Behrens J (2000) Control of beta-catenin signaling in tumor development. Ann NY Acad Sci 910:21–33 discussion 33-25
Morin PJ (1999) β-catenin signaling and cancer. BioEssays: news and reviews in molecular, cellular and developmental biology 21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454. doi:10.1038/nrc822
Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M (2007) Vimentin and epithelial-mesenchymal transition in human breast cancer–observations in vitro and in vivo. Cells Tissues Organs 185:191–203. doi:10.1159/000101320
Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J (2011) Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 30:1436–1448. doi:10.1038/onc.2010.509
Webster DR, Gundersen GG, Bulinski JC, Borisy GG (1987) Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA 84:9040–9044
Mialhe A, Lafanechere L, Treilleux I, Peloux N, Dumontet C, Bremond A, Panh MH, Payan R, Wehland J, Margolis RL, Job D (2001) Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res 61:5024–5027
Kreis TE (1987) Microtubules containing detyrosinated tubulin are less dynamic. EMBO J 6:2597–2606
Peris L, Wagenbach M, Lafanechere L, Brocard J, Moore AT, Kozielski F, Job D, Wordeman L, Andrieux A (2009) Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol 185:1159–1166. doi:10.1083/jcb.200902142
Barak V, Goike H, Panaretakis KW, Einarsson R (2004) Clinical utility of cytokeratins as tumor markers. Clin Biochem 37:529–540. doi:10.1016/j.clinbiochem.2004.05.009
Acknowledgments
This work was supported by Grants to G Sabnis (KG10037) from Susan G Komen for the Cure.
Conflict of interest
We do not have any relevant conflict of interest. Syndax Pharmaceuticals (MA, USA) provided entinostat and letrozole used in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shah, P., Gau, Y. & Sabnis, G. Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res Treat 143, 99–111 (2014). https://doi.org/10.1007/s10549-013-2784-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2784-7