Abstract
To investigate the expression levels of CXCL10 and CXCR3 in tumors from breast cancer patients randomized to adjuvant tamoxifen treatment or no endocrine treatment, in order to further study the connection to prognosis and prediction of tamoxifen treatment outcome. Immunohistochemistry on tissue microarrays from 912 breast cancer patients randomized to tamoxifen or no endocrine treatment. CXCR3 status was found to be a prognostic tool in predicting distant recurrence, as well as reduced breast cancer-specific survival. In patients with estrogen receptor (ER)-positive tumors, tumors with strong CXCL10 levels had improved effect of tamoxifen treatment in terms of local recurrence-free survival [risk ratio (RR) 0.46 (95 % CI 0.25–0.85, P = 0.01)] compared with patients with tumors expressing weak CXCL10 expression. Further, patients with ER-positive tumors with strong CXCR3 expression had an improved effect of tamoxifen in terms of breast cancer-specific survival [RR 0.34 (95 % CI 0.19–0.62, P < 0.001)] compared with the group with weak CXCR3 levels [RR 1.33 (95 % CI 0.38–4.79, P = 0.65)]. We show here for the first time that CXCL10 and CXCR3 expression are both predictors of favorable outcome in patients treated with tamoxifen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common form of cancer among women. There is increasing evidence suggesting the relevance of interactions between microenvironment and mammary epithelial for proliferation, differentiation, survival, and invasion. The mechanisms behind these interactions are to a large extent unknown, but inflammatory cells are suggested to play an important role in breast cancer. This work focuses on the chemokine, C–X–C motif ligand 10 (CXCL10), also known as γ-interferon-induced protein of 10 kDa (IP 10). CXCL10s ability to recruit T-cells is well known [1–5], and its role in cancer is established [6–10]. CXCL10 is under the positive regulation of interferon-α and -γ [10, 11], interleukin-10 [12], interleukin-1α, and tumor necrosis factor (TNF)-α [13]. In a murine model, mice injected with CXCL10-expressing tumor cells, CXCL10 prevented the formation of new tumors, and mediated the regression of existing ones [14]. The primary receptor for CXCL10 is C–X–C motif receptor 3 (CXCR3). CXCR3 has two reported isoforms. CXCR3-A, the originally discovered isoform, mediates chemotaxis of immune and cancer cells, as well as proliferative signaling and promotion of angiogenesis [15–17]. CXCR3-B inhibits cell motility, reduces proliferative signaling and inhibits angiogenesis [15–18]. Increase of total CXCR3 has been observed in breast cancer, but no analysis of CXCR3-A and CXCR3-B ratio has been conducted [8, 15, 16].
The relevance and function of CXCL10 and CXCR3 in terms of prognosis and breast cancer treatment prediction is poorly understood. Estrogen has been reported to reduce CXCL10 expression [19] and tamoxifen may enhance the immune response [1, 2, 20, 21]. The purpose of this study was to investigate the expression levels of CXCL10 and CXCR3 in tumors from breast cancer patients randomized to adjuvant tamoxifen treatment or no endocrine treatment, to further study the connection to prognosis and prediction of tamoxifen treatment outcome in low risk and low stage patients.
Materials and methods
This study was designed and presented with regard to the reporting recommendations for tumor marker prognostic studies (REMARK) guidelines [22].
Patients
This retrospective cohort study was conducted using tumor material from a randomized tamoxifen trial conducted in 1976–1990 in Stockholm Sweden composed of 1,780 patients. Results and details of the trial were previously described [23]. All patients were postmenopausal with tumors ≤30 mm and were negative for axillary lymph node involvement (N0). The patients received either breast conserving surgery followed by radiation treatment with a dose of 50 Gy with 2 Gy per fraction 5 days weekly, for about 5 weeks, or radical mastectomy. After surgery, patients were randomized to tamoxifen 40 mg daily or no endocrine treatment. After 2 years of tamoxifen treatment, most disease-free patients were randomized to tamoxifen for an additional 3 years or no further therapy. Tumor material from 912 women was available for the current investigation. The mean follow-up period for patients in the study was 18 years. The trial design and protocol was approved by the Research Ethics Committee at the Karolinska Institute (dnr 97–451, with amendments) [23]. In order to conduct tissue microarray analysis, a pathologist chose representative parts of the tumors. Three cores per patient with a diameter of 0.8 mm were chosen and transferred to a paraffin block using a manual arrayer (Beecher Instruments, Sun Prairie, WI). From the sample blocks sections were cut and placed on slides, forming the basis of the tissue microarray. A flow chart of patients included in the initial tamoxifen trial and further included in the current analysis is shown in Fig. 1. Estrogen receptor (ER) and progesterone receptor (PgR) status were determined with cut-off levels at 10 % of positively stained tumor cell nuclei, cytosol measurements were used in the case of missing immunohistochemical data, with a cut-off of 0.05 fmol/μg DNA [23]. HER2 expression score 0–3 was previously described [24].
Immunostaining for CXCL10 and CXCR3 expression
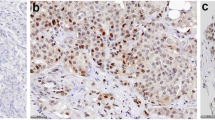
Tissue microarray sample slides were heated to 60 °C for 12 h and stored at −70 °C. Following thawing, the sample slides were deparaffinized with Tissue Clear (HistoLab, Göteborg, Sweden). The sample slides were washed with decreasing concentrations of ethanol followed by water, after which they were placed in DIVA-buffer (BioCare, Concord, CA) inside a decloaking chamber (BioCare), heated to 120 °C, cooled to 90 °C and then left in room temperature. The sample slides were washed with PBS containing 0.1 % bovine serum albumin (BSA) and Protein Block (Spring Bioscience, Pleasanton, CA). Primary monoclonal anti-CXCR3 antibody [2Ar1] at 500 ng/ml (Abcam, Cambridge, UK), primary polyclonal rabbit anti-CXCL10 antibody at 111 ng/ml (ab9807, Abcam) or control IgG mouse antibody at 500 or 111 ng/ml (DAKO, Glostrup, Denmark) were added and the sample slides were incubated overnight at 4 °C. Envision secondary anti-mouse antibody conjugated to HRP (DAKO) was added, thereafter the sample slides were incubated in 3,3′-diaminobenzidine tetrahydrochloride (DAB) with hydrogen peroxide and counterstained with hematoxylin (BioRad, Hercules, CA), rehydrated and fixated with Mount-X (HistoLab) mounting solution. Grading was done using a Leica LB30T microscope (Leica Microsystems, Wetzlar, Germany). Sample scoring was done without evaluators’ knowledge of clinical or pathological data for patients, using a 0–3 scaling system, − indicating no staining, + indicating weak expression, ++ indicating moderate expression, and +++ indicating a strong expression. Only tumor cells were analyzed. Two individual evaluators judged all slides independently. Representative slides for each intensity were photographed using an Axio Lab A1 microscope with an AxioCam ICc5 camera (Zeiss, Oberkochen, Germany), all images were acquired using image acquire software Zen 2012 blue edition (Zeiss).
Statistical analysis
The relationships between grouped variables were analyzed using Spearman’s rank order correlation. To compensate for multiple testing P < 0.01 was set as significant. The survival curves were produced according to the lifetable method described by Kaplan and Meier and differences between groups were evaluated with log-rank tests. Patients with missing data were excluded. Univariate and multivariate analysis were conducted using Cox proportional hazards regression and P < 0.05 was considered significant. End points used were breast cancer-specific mortality, defined as when patients had a local or distant recurrence or when so stated by the Swedish cause of death registry. Local recurrence defined as a relapse on either chest wall or in a regional lymph node or distant recurrence, defined as the remaining metastatic events. The statistical package Statistica 10.0 (StatSoft Scandinavia, Uppsala, Sweden) was used for all calculations.
Results
CXCL10 and CXCR3 expression in relation to tumor characteristics
Out of 912 patients, data for CXCL10 expression were acquired from 793 cases. In our material, 51 (6 %) of the patients showed no CXCL10 expression, 183 (23 %) weak expression, 224 (28 %) moderate expression, and 335 (42 %) strong expression (Fig. 2). Data for CXCR3 expression were acquired from 735 cases and 29 (4 %) patients showed no expression, 121 (17 %) weak expression, 282 (38 %) moderate expression, and 303 (41 %) strong CXCR3 expression (Fig. 3). CXCR3 expression was positively correlated with HER2 expression (P < 0.001), no correlation between CXCL10 and CXCR3 was found, and no correlation between ER, PgR nor tumor size was detected for CXCL10 or CXCR3 (Tables 1, 2).
CXCL10 and CXCR3 are predictive factors of tamoxifen treatment in breast cancer
In the following analysis conducted to investigate the tamoxifen prediction value of CXCL10 and CXCR3, the patients with ER-positive tumors were divided in groups with tumors showing low (no or weak) or high (moderate or strong) expression. Patients with strong tumoral CXCL10 expression who received tamoxifen treatment had significantly improved local recurrence-free survival compared with patients who did not receive tamoxifen [risk ratio (RR) 0.46 (95 % CI 0.25–0.85, P = 0.01)] (Fig. 4a). Patients treated with tamoxifen who had low tumoral expression of CXCL10 showed no significant effect of treatment on local recurrence-free survival [RR 1.15 (95 % CI 0.46–2.85, P = 0.77)] (Fig. 4b). No predictive value for CXCL10 was shown in terms of breast cancer-specific survival or distant recurrence-free survival (data not shown).
Patients with strong tumoral CXCR3 expression showed benefit from tamoxifen treatment with regards to the end point breast cancer-specific survival [RR 0.34 (95 % CI 0.19–0.62, P < 0.001)], local recurrence-free survival [RR 0.35 (95 % CI 0.22–0.58, P < 0.001)], and distant recurrence-free survival [RR 0.44 (95 % CI 0.24–0.81, P = 0.009)] compared with patients who did not receive tamoxifen treatment (Fig. 5a–c). Patients with tumors that had low CXCR3 expression showed no significant effect of tamoxifen treatment for breast cancer-specific survival [RR 1.33 (95 % CI 0.38–4.79, P = 0.65)], local recurrence-free survival [RR 1.24 (95 % CI 0.41–4.07, P = 0.68)], or distant recurrence-free survival [RR 1.21 (95 % CI 0.42–3.48, P = 0.72)] (Fig. 5d–f). The interaction test was significant (P = 0.04) with distant recurrence-free survival as end point.
Survival curves for tamoxifen-treated patients, grouped according to tumoral CXCR3 expression. All patient tumors are estrogen receptor positive. a–c Patients with moderate/high tumoral CXCR3 expression and d–f patients with no/low tumoral CXCR3 expression. a, d Breast cancer-specific survival. b, e Local recurrence-free survival. c, f Distant recurrence-free survival
CXCR3, but not CXCL10 is a prognostic factor in breast cancer
In the following analysis, the ability of CXCL10 and CXCR3 as prognostic factors in tamoxifen untreated patients was analyzed. Patients were organized in groups with tumors showing low (no and weak) or high (moderate or strong) expression. CXCL10 was not a prognostic marker for breast cancer-specific survival, local recurrence-free survival, or distant recurrence-free survival. However, CXCR3 was shown to be a prognostic marker concerning breast cancer-specific survival [RR 1.48 (95 % CI 1–2.19, P = 0.05)] (Fig. 6a), and distant recurrence-free survival [RR 1.40 (95 % CI 1.02–1.92, P = 0.036)] (Fig. 6c). Multivariate analysis supported this for both breast cancer-specific survival [RR 1.59 (95 % CI 1–2.53, P = 0.47)] and distant recurrence-free survival [RR 1.61 (95 % CI 1.09–2.38, P = 0.016)], Table 3. CXCR3 was not a prognostic marker for local recurrence-free survival (Fig. 6b).
Discussion
We report for the first time that patients with ER-positive tumors and high tumoral CXCL10 expression have a markedly improved effect of tamoxifen compared with patients with ER-positive tumors and low tumoral CXCL10 expression in terms of local recurrence-free survival. The observed improvement of the tamoxifen effect in patients with high tumoral CXCL10 expression could be a result of the recruitment of T effector cells to sites of expression [1, 2]. T-cells have been shown to mediate antitumor activity and protection of the tissue from the recurrence of tumor cells [2, 20]. In a murine model, Aronica et al. [25] show that CXCL10 can prevent estrogen-induced tumor formation and estrogen-induced growth of tumor cells via inhibition of vascular endothelial growth factor (VEGF) signaling. Taken together with the antiestrogenic properties of tamoxifen, which results in the inhibition VEGF signaling, and it is possible that the synergic inhibition of VEGF by CXCL10 and tamoxifen could give an improved effect compared with tamoxifen alone [25].
In patients who did not receive endocrine treatment there was no impact based on CXCL10 expression in terms of any of our tested endpoints. This could be attributed to the relatively early stage of breast cancer in our material, with small tumors and no nodal involvement, thus once the tumor is removed and the chemotactic recruitment is weakened, the CXCL10 might no longer be able to predict patient clinical outcome on its own.
Patients with ER-positive tumors with a high tumoral CXCR3 expression had improved effect of tamoxifen when compared with patients with ER-positive tumors and low CXCR3 expression. CXCR3 cellular membrane expression is limited to the G2/M phase of the cell cycle [15, 18, 26, 27]. As a result of ER-α inhibition by tamoxifen, the amount of cells in G2/M phase would likely decrease, reducing CXCR3-mediated signaling. Indeed, Janis et al. found that CXCR3 levels decreased following tamoxifen stimulation [28]. Thus, tumor cells dependent on CXCR3 signaling for directing migration, metastasis, or proliferation would find themselves bereft of both ER-α signaling and CXCR3 signaling.
We report that high CXCR3 expression is a good indicator of tamoxifen response, while in patients who receive no endocrine treatment high tumoral CXCR3 expression was associated with a worse patient outcome. The significant impact of high CXCR3 expression in relation to prognosis of patient outcome was seen for distant recurrence-free survival, which may be attributed to the ability of CXCR3 to mediate metastasis, shown in several other forms of cancer [10, 15–17, 29–31]. Our prognostic data from CXCR3 in breast cancer are supported by Ma et al. [16], who showed that high CXCR3 expression levels are associated with a worsened prognosis. The patient material used by Ma et al. is small, but interestingly, they found the correlation between CXCR3 level and patient outcome only in the subset of patients which, like our patients, have no nodal involvement, indicating that CXCR3 might be an important aspect of early metastasis. No combination of the expressions of CXCR3 and CXCL10 provides any insight into patient prognosis (data not shown), a result in line with observations in a previous study by Mulligan et al. [32]. These data taken together with the clinical importance of distant recurrence in terms of patient point toward a role of CXCR3, but not CXCL10 in prognosis of patient outcome, in terms of metastatic potential and overall survival.
We found no correlation between CXCL10 and CXCR3 levels, which contradicts previous findings by Mulligan et al. This difference could be attributed to differences in the materials. Our material is selected from low stage cancers, while Mulligan used heterogeneous material with varying stage, nodal involvement and grade, in addition over a hundred of these patients had mutations in either BRCA1 or BRCA2 [32]. We found a correlation between HER2 and CXCR3 expression. Nejatollahi et al. describe that after treatment with single chain fragment variable antibodies targeting HER2, the protein levels of both HER2 and CXCR3 were reduced [33].
CXCR3 has two primary CXCL10-binding isoforms reported in the literature, CXCR3-A and CXCR3-B. Several claims have been made to CXCR3-A- and CXCR3-B-specific effects, with CXCR3-A-supporting proliferative migratory effects and CXCR3-B-antiproliferative and antimigratory effects [15–18]. However, due to a large degree of homology between the two proteins, with CXCR3-B having a 47 amino acid insert as the only area of dissimilarity to CXCR3-A [17], there are no publicly available antibodies which are subtype specific. Similar problems are present utilizing mRNA quantification with qPCR, since the region surrounding the area of dissimilarity is poorly suited for primers or probes, and no reported [17, 18, 34, 35] or commercially available primer/probe set can reliably and with good efficiency quantify mRNA from CXCR3-A and not include false positives from CXCR3-B mRNA.
Conclusion
Herein, we show that in ER-positive patients both the chemokine CXCL10 and its primary receptor CXCR3 can be used to predict improved patient treatment response to tamoxifen compared to when only ER is used as a clinical marker. We propose that this pathway be further evaluated for the use in clinical setting to clearly define the role these markers have in the outcome of the patient in different patient subsets, and whether treatment targeting the CXCL10/CXCR3 axis could provide further benefit to these patients. We also found that CXCR3 status can be a prognostic tool in predicting metastasis, as well as reduced overall survival.
References
Groom JR, Luster AD (2011) CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89(2):207–215. doi:10.1038/icb.2010.158
Kajitani K, Tanaka Y, Arihiro K, Kataoka T, Ohdan H (2012) Mechanistic analysis of the antitumor efficacy of human natural killer cells against breast cancer cells. Breast Cancer Res Treat 134(1):139–155. doi:10.1007/s10549-011-1944-x
Luster AD, Leder P (1993) IP-10, a –C–X–C– chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med 178(3):1057–1065
Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ (1993) Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med 177(6):1809–1814
Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K (1998) Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 187(12):2009–2021
Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM (1996) Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med 184(3):981–992
Giuliani N, Bonomini S, Romagnani P, Lazzaretti M, Morandi F, Colla S, Tagliaferri S, Lasagni L, Annunziato F, Crugnola M, Rizzoli V (2006) CXCR3 and its binding chemokines in myeloma cells: expression of isoforms and potential relationships with myeloma cell proliferation and survival. Haematologica 91(11):1489–1497
Goldberg-Bittman L, Neumark E, Sagi-Assif O, Azenshtein E, Meshel T, Witz IP, Ben-Baruch A (2004) The expression of the chemokine receptor CXCR3 and its ligand, CXCL10, in human breast adenocarcinoma cell lines. Immunol Lett 92(1–2):171–178
Lo BK, Yu M, Zloty D, Cowan B, Shapiro J, McElwee KJ (2010) CXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomas. Am J Pathol 176(5):2435–2446
Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, Witz IP, Ben-Baruch A (2007) CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res 67(7):3396–3405
Persano L, Moserle L, Esposito G, Bronte V, Barbieri V, Iafrate M, Gardiman MP, Larghero P, Pfeffer U, Naschberger E, Sturzl M, Indraccolo S, Amadori A (2009) Interferon-alpha counteracts the angiogenic switch and reduces tumor cell proliferation in a spontaneous model of prostatic cancer. Carcinogenesis 30(5):851–860
Dorsey R, Kundu N, Yang Q, Tannenbaum CS, Sun H, Hamilton TA, Fulton AM (2002) Immunotherapy with interleukin-10 depends on the CXC chemokines inducible protein-10 and monokine induced by IFN-gamma. Cancer Res 62(9):2606–2610
Hu J, You S, Li W, Wang D, Nagpal ML, Mi Y, Liang P, Lin T (1998) Expression and regulation of interferon-gamma-inducible protein 10 gene in rat Leydig cells. Endocrinology 139(8):3637–3645
Chu Y, Yang X, Xu W, Wang Y, Guo Q, Xiong S (2007) In situ expression of IFN-gamma-inducible T cell alpha chemoattractant in breast cancer mounts an enhanced specific anti-tumor immunity which leads to tumor regression. Cancer Immunol Immunother 56(10):1539–1549. doi:10.1007/s00262-007-0296-1
Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S (2006) Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res 66(19):9509–9518
Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A (2009) CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther 8(3):490–498
Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P (2003) An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197(11):1537–1549
Wu Q, Dhir R, Wells A (2012) Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol Cancer 11(1):3. doi:10.1186/1476-4598-11-3
Aronica SM, Fanti P, Kaminskaya K, Gibbs K, Raiber L, Nazareth M, Bucelli R, Mineo M, Grzybek K, Kumin M, Poppenberg K, Schwach C, Janis K (2004) Estrogen disrupts chemokine-mediated chemokine release from mammary cells: implications for the interplay between estrogen and IP-10 in the regulation of mammary tumor formation. Breast Cancer Res Treat 84(3):235–245. doi:10.1023/B:BREA.0000019961.59306.f6
Curigliano G (2011) Immunity and autoimmunity: revising the concepts of response to breast cancer. Breast 20(Suppl 3):S71–S74. doi:10.1016/S0960-9776(11)70298-3
Schild-Hay LJ, Leil TA, Divi RL, Olivero OA, Weston A, Poirier MC (2009) Tamoxifen induces expression of immune response-related genes in cultured normal human mammary epithelial cells. Cancer Res 69(3):1150–1155. doi:10.1158/0008-5472.CAN-08-2806
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of NCIEWGoCD (2006) Reporting recommendations for tumor marker prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235. doi:10.1007/s10549-006-9242-8
Rutqvist LE, Johansson H, Stockholm Breast Cancer Study Group (2007) Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol 46(2):133–145
Jansson A, Delander L, Gunnarsson C, Fornander T, Skoog L, Nordenskjold B, Stal O (2009) Ratio of 17HSD1 to 17HSD2 protein expression predicts the outcome of tamoxifen treatment in postmenopausal breast cancer patients. Clin Cancer Res 15(10):3610–3616
Aronica SM, Raiber L, Hanzly M, Kisela C (2009) Antitumor/antiestrogenic effect of the chemokine interferon inducible protein 10 (IP-10) involves suppression of VEGF expression in mammary tissue. J Interferon Cytokine Res 29(2):83–92. doi:10.1089/jir.2008.0034
Aksoy MO, Yang Y, Ji R, Reddy PJ, Shahabuddin S, Litvin J, Rogers TJ, Kelsen SG (2006) CXCR3 surface expression in human airway epithelial cells: cell cycle dependence and effect on cell proliferation. Am J Physiol Lung Cell Mol Physiol 290(5):L909–L918
Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M (2001) Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Investig 107(1):53–63
Janis K, Hoeltke J, Nazareth M, Fanti P, Poppenberg K, Aronica SM (2004) Estrogen decreases expression of chemokine receptors, and suppresses chemokine bioactivity in murine monocytes. Am J Reprod Immunol 51(1):22–31
Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM (2004) Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res 64(11):4010–4017
Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, Martini V, Birnbaum D, Scoazec JY, Abello J, Al Saati T, Johnson MG, Sullivan TJ, Medina JC, Collins TL, Schmid-Alliana A, Schmid-Antomarchi H (2009) Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer 100(11):1755–1764. doi:10.1038/sj.bjc.6605078
Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M, Taketo MM (2007) Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 26(32):4679–4688. doi:10.1038/sj.onc.1210267
Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O’Malley FP, Ohashi PS, Andrulis IL (2013) Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res 19(2):336–346. doi:10.1158/1078-0432.CCR-11-3314
Nejatollahi F, Ranjbar R, Younesi V, Asgharpour M (2013) Deregulation of HER2 downstream signaling in breast cancer cells by a cocktail of anti-HER2 scFvs. Oncol Res 20(8):333–340. doi:10.3727/096504013X13657689382734
Gacci M, Serni S, Lapini A, Vittori G, Alessandrini M, Nesi G, Palli D, Carini M (2009) CXCR3-B expression correlates with tumor necrosis extension in renal cell carcinoma. J Urol 181(2):843–848. doi:10.1016/j.juro.2008.10.063
Datta D, Banerjee P, Gasser M, Waaga-Gasser AM, Pal S (2010) CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1. J Biol Chem 285(47):36842–36848
Acknowledgments
We would like to thank the patients that participated in this study. We would also like to thank the Swedish Science Council (www.vr.se) and the Cancer Foundation (www.cancerfonden.se) for the funding which supported this project. Thanks to Agieszka Kot for her assistance in staining and evaluating the CXCL10 staining. We also want to thank Lambert Skoog at the Department of Cytology, Karolinska University Hospital, Stockholm, Sweden, for his assistance in gathering the patient material and for the assistance staining and evaluating ER and HER2. We wish to thank Birgitta Holmlund for her help in constructing the tissue microarrays.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hilborn, E., Sivik, T., Fornander, T. et al. C–X–C ligand 10 and C–X–C receptor 3 status can predict tamoxifen treatment response in breast cancer patients. Breast Cancer Res Treat 145, 73–82 (2014). https://doi.org/10.1007/s10549-014-2933-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2933-7