Abstract
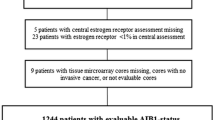
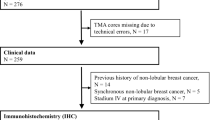
AIB1 (amplified in breast cancer 1) is an estrogen receptorα (ERα) co-activator, known to be amplified and overexpressed in a fraction of breast cancers. It has been linked to prognosis and tamoxifen resistance. However, results have been ambiguous. The different functions of AIB1 in ERα-positive and -negative disease are poorly understood. Therefore, we analyzed the clinical significance of AIB1 in breast cancer with respect to ERα-status and characterized the subgroups. 2,197 breast carcinomas sampled on a pre-existing tissue microarray (TMA) were analyzed for AIB1 expression and amplification by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). Results: AIB1 expression was detected in 60 % of the tumors. It was associated with tumor size (p = 0.003), high histological grade (p < 0.0001), poor disease-specific, and overall survival (p = 0.0018 and p = 0.003). There was a strong inverse relationship between AIB1 and ERα expression (p < 0.0001). AIB1 overexpression was associated with increased Ki67 labeling index (p < 0.0001), even if analyzed for different ER expression levels. AIB1 amplification was found in 11 % of the carcinomas. It was associated with high histological grade (p = 0.0012), lymph node involvement (p = 0.0163), and poor disease-specific survival (p = 0.0032) but not with overall survival (p = 0.1672) or ER status (p = 0.4456). If ER-positive tumors were stratified according to their AIB1 amplification status, there was a significant worse disease-specific survival in cases showing AIB1 amplification (p = 0.0017). AIB1 expression is associated with unfavorable prognosis and tumor phenotype. It seems to unfold its oncogenic potential at least in part independent from its role as an ERα co-activator. AIB1 has an impact on cell cycle regulation in ERα-positive as well as ERα-negative tumors. Furthermore, AIB1 amplification characterizes a subgroup of ERα-positive breast cancer with worse outcome. Therefore, AIB1 might be helpful to identify those ERα-positive breast cancers patients who are candidates for adjuvant chemotherapy.





Similar content being viewed by others
References
Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai MJ, O’Malley BW (1997) Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res 52:141–164 discussion 164–145
Torchia J, Glass C, Rosenfeld MG (1998) Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol 10(3):373–383
Parker MG (1998) Transcriptional activation by oestrogen receptors. Biochem Soc Symp 63:45–50
Schiff R, Massarweh S, Shou J, Osborne CK (2003) Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res 9(1 Pt 2):447S–454S
Martini PG, Katzenellenbogen BS (2003) Modulation of estrogen receptor activity by selective coregulators. J Steroid Biochem Mol Biol 85(2–5):117–122
Guan XY, Xu J, Anzick SL, Zhang H, Trent JM, Meltzer PS (1996) Hybrid selection of transcribed sequences from microdissected DNA: isolation of genes within amplified region at 20q11-q13.2 in breast cancer. Cancer Res 56(15):3446–3450
Li J, O’Malley BW, Wong J (2000) p300 requires its histone acetyltransferase activity and SRC-1 interaction domain to facilitate thyroid hormone receptor activation in chromatin. Mol Cell Biol 20(6):2031–2042
Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM (1997) Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90(3):569–580
Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O’Malley BW (1997) Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389(6647):194–198
Liao L, Kuang SQ, Yuan Y, Gonzalez SM, O’Malley BW, Xu J (2002) Molecular structure and biological function of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J Steroid Biochem Mol Biol 83(1–5):3–14
Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS (1997) AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277(5328):965–968
Bautista S, Valles H, Walker RL, Anzick S, Zeillinger R, Meltzer P, Theillet C (1998) In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res 4(12):2925–2929
Bouras T, Southey MC, Venter DJ (2001) Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res 61(3):903–907
List HJ, Reiter R, Singh B, Wellstein A, Riegel AT (2001) Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res Treat 68(1):21–28
Murphy LC, Simon SL, Parkes A, Leygue E, Dotzlaw H, Snell L, Troup S, Adeyinka A, Watson PH (2000) Altered expression of estrogen receptor coregulators during human breast tumorigenesis. Cancer Res 60(22):6266–6271
Iwase H, Omoto Y, Toyama T, Yamashita H, Hara Y, Sugiura H, Zhang Z (2003) Clinical significance of AIB1 expression in human breast cancer. Breast Cancer Res Treat 80(3):339–345
Kirkegaard T, McGlynn LM, Campbell FM, Muller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JM (2007) Amplified in breast cancer 1 in human epidermal growth factor receptor—positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res 13(5):1405–1411
Dihge L, Bendahl PO, Grabau D, Isola J, Lovgren K, Ryden L, Ferno M (2007) Epidermal growth factor receptor (EGFR) and the estrogen receptor modulator amplified in breast cancer (AIB1) for predicting clinical outcome after adjuvant tamoxifen in breast cancer. Breast Cancer Res Treat 109(2):255–262
Kurebayashi J, Otsuki T, Kunisue H, Tanaka K, Yamamoto S, Sonoo H (2000) Expression levels of estrogen receptor-alpha, estrogen receptor-beta, coactivators, and corepressors in breast cancer. Clin Cancer Res 6(2):512–518
Spears M, Oesterreich S, Migliaccio I, Guiterrez C, Hilsenbeck S, Quintayo MA, Pedraza J, Munro AF, Thomas JS, Kerr GR, Jack WJ, Kunkler IH, Cameron DA, Chetty U, Bartlett JM (2011) The p160 ER co-regulators predict outcome in ER negative breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-011-1426-1
Ruiz C, Seibt S, Al Kuraya K, Siraj AK, Mirlacher M, Schraml P, Maurer R, Spichtin H, Torhorst J, Popovska S, Simon R, Sauter G (2006) Tissue microarrays for comparing molecular features with proliferation activity in breast cancer. Int J Cancer 118(9):2190–2194
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235. doi:10.1007/s10549-006-9242-8
Wagner U, Bubendorf L, Gasser T, Moch H, Görög J, Mihatsch M, Waldman F, Sauter G (1997) Chromosome 8p deletions are associated with invasive tumor growth in urinary bladder cancer. Am J Pathol 151:753–759
Simon R, Nocito A, Hubscher T, Bucher C, Torhorst J, Schraml P, Bubendorf L, Mihatsch MM, Moch H, Wilber K, Schotzau A, Kononen J, Sauter G (2001) Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst 93(15):1141–1146
Simon R, Richter J, Wagner U, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knonagel H, Rist M, Wilber K, Anabitarte M, Hering F, Hardmeier T, Schonenberger A, Flury R, Jager P, Fehr JL, Schraml P, Moch H, Mihatsch MJ, Gasser T, Sauter G (2001) High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res 61(11):4514–4519
Simon R, Struckmann K, Schraml P, Wagner U, Forster T, Moch H, Fijan A, Bruderer J, Wilber K, Mihatsch MJ, Gasser T, Sauter G (2002) Amplification pattern of 12q13-q15 genes (MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene 21(16):2476–2483. doi:10.1038/sj.onc.1205304
Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, Inazawa J (2003) Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer 98(1):18–23
Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF (2003) Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat 78(2):193–204
Alkner S, Bendahl PO, Grabau D, Lovgren K, Stal O, Ryden L, Ferno M (2010) AIB1 is a predictive factor for tamoxifen response in premenopausal women. Ann Oncol 21(2):238–244. doi:10.1093/annonc/mdp293
Harigopal M, Heymann J, Ghosh S, Anagnostou V, Camp RL, Rimm DL (2009) Estrogen receptor co-activator (AIB1) protein expression by automated quantitative analysis (AQUA) in a breast cancer tissue microarray and association with patient outcome. Breast Cancer Res Treat 115(1):77–85. doi:10.1007/s10549-008-0063-9
Lee K, Lee A, Song BJ, Kang CS (2011) Expression of AIB1 protein as a prognostic factor in breast cancer. World J Surg Oncol 9:139. doi:10.1186/1477-7819-9-139
Cuny M, Kramar A, Courjal F, Johannsdottir V, Iacopetta B, Fontaine H, Grenier J, Culine S, Theillet C (2000) Relating genotype and phenotype in breast cancer: an analysis of the prognostic significance of amplification at eight different genes or loci and of p53 mutations. Cancer Res 60(4):1077–1083
Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R (2003) Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95(5):353–361
Zhao W, Zhang Q, Kang X, Jin S, Lou C (2009) AIB1 is required for the acquisition of epithelial growth factor receptor-mediated tamoxifen resistance in breast cancer cells. Biochem Biophys Res Commun 380(3):699–704. doi:10.1016/j.bbrc.2009.01.155
Reiter R, Oh AS, Wellstein A, Riegel AT (2004) Impact of the nuclear receptor coactivator AIB1 isoform AIB1-Delta3 on estrogenic ligands with different intrinsic activity. Oncogene 23(2):403–409. doi:10.1038/sj.onc.12072021207202
Louie MC, Zou JX, Rabinovich A, Chen HW (2004) ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24(12):5157–5171. doi:10.1128/MCB.24.12.5157-5171.200424/12/5157
Yan J, Tsai SY, Tsai MJ (2006) SRC-3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol Sin 27(4):387–394. doi:10.1111/j.1745-7254.2006.00315.x
Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M (2004) High tumor incidence and activation of the PI3 K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6(3):263–274. doi:10.1016/j.ccr.2004.06.027S1535610804002193
Werbajh S, Nojek I, Lanz R, Costas MA (2000) RAC-3 is a NF-kappa B coactivator. FEBS Lett 485(2–3):195–199. doi:S0014-5793(00)02223-7
Tilli MT, Reiter R, Oh AS, Henke RT, McDonnell K, Gallicano GI, Furth PA, Riegel AT (2005) Overexpression of an N-terminally truncated isoform of the nuclear receptor coactivator amplified in breast cancer 1 leads to altered proliferation of mammary epithelial cells in transgenic mice. Mol Endocrinol 19(3):644–656. doi:10.1210/me.2004-0106
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96(12):926–935
Lahusen T, Henke RT, Kagan BL, Wellstein A, Riegel AT (2009) The role and regulation of the nuclear receptor co-activator AIB1 in breast cancer. Breast Cancer Res Treat 116(2):225–237. doi:10.1007/s10549-009-0405-2
Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, Ittmann M, Tsai SY, Tsai MJ (2005) SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res 65(17):7976–7983. doi:10.1158/0008-5472.CAN-04-4076
Xu FP, Xie D, Wen JM, Wu HX, Liu YD, Bi J, Lv ZL, Zeng YX, Guan XY (2007) SRC-3/AIB1 protein and gene amplification levels in human esophageal squamous cell carcinomas. Cancer Lett 245(1–2):69–74. doi:10.1016/j.canlet.2005.12.030
Luo JH, Xie D, Liu MZ, Chen W, Liu YD, Wu GQ, Kung HF, Zeng YX, Guan XY (2008) Protein expression and amplification of AIB1 in human urothelial carcinoma of the bladder and overexpression of AIB1 is a new independent prognostic marker of patient survival. Int J Cancer 122(11):2554–2561. doi:10.1002/ijc.23399
Nonet GH, Stampfer MR, Chin K, Gray JW, Collins CC, Yaswen P (2001) The ZNF217 gene amplified in breast cancers promotes immortalization of human mammary epithelial cells. Cancer Res 61(4):1250–1254
Collins C, Rommens JM, Kowbel D, Godfrey T, Tanner M, Hwang SI, Polikoff D, Nonet G, Cochran J, Myambo K, Jay KE, Froula J, Cloutier T, Kuo WL, Yaswen P, Dairkee S, Giovanola J, Hutchinson GB, Isola J, Kallioniemi OP, Palazzolo M, Martin C, Ericsson C, Pinkel D, Albertson D, Li WB, Gray JW (1998) Positional cloning of ZNF217 and NABC1: genes amplified at 20q13.2 and overexpressed in breast carcinoma. Proc Natl Acad Sci U S A 95(15):8703–8708
Sen S, Zhou H, White RA (1997) A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene 14(18):2195–2200
Forozan F, Mahlamaki EH, Monni O, Chen Y, Veldman R, Jiang Y, Gooden GC, Ethier SP, Kallioniemi A, Kallioniemi OP (2000) Comparative genomic hybridization analysis of 38 breast cancer cell lines: a basis for interpreting complementary DNA microarray data. Cancer Res 60(16):4519–4525
Barlund M, Monni O, Weaver JD, Kauraniemi P, Sauter G, Heiskanen M, Kallioniemi OP, Kallioniemi A (2002) Cloning of BCAS3 (17q23) and BCAS4 (20q13) genes that undergo amplification, overexpression, and fusion in breast cancer. Genes Chromosom Cancer 35(4):311–317. doi:10.1002/gcc.10121
Lim E, Winer EP (2011) Adjuvant chemotherapy in luminal breast cancers. Breast 20(Suppl 3):S128–S131. doi:10.1016/S0960-9776(11)70309-5
Acknowledgments
The authors appreciate the excellent technical support of Christina Koop, Sylvia Schnöger, and Sasha Eghtessadi.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burandt, E., Jens, G., Holst, F. et al. Prognostic relevance of AIB1 (NCoA3) amplification and overexpression in breast cancer. Breast Cancer Res Treat 137, 745–753 (2013). https://doi.org/10.1007/s10549-013-2406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2406-4