Abstract
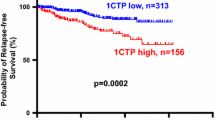
Bone is the most common site of metastasis of breast cancer, affecting most women with advanced disease. Procollagen type I N-terminal propeptide (P1NP), osteocalcin, CTX, and IL-6 are markers of bone turnover. Our objective was to determine whether serum levels of these proteins have clinical utility as predictors of breast cancer metastasis to bone. Blood was collected before treatment from 164 patients with stage I–III breast cancer from September 2001 to December 2008. Serum levels of P1NP, CTX, IL-6, and OC were measured using an automated immunoassay system. Correlations of the levels of these markers with time to bone metastasis development and with overall survival (OS) rate were assessed using Cox proportional hazards regression analysis and the Kaplan–Meier method. Fifty-five patients with stage I–III disease at the time of blood sample collection subsequently experienced metastasis to bone. A baseline P1NP level of at least 75 ng/mL predicted increased risk of bone metastasis (hazard ratio, 2.7 [95 % confidence interval, 1.2–6.0]; P = 0.031) and a poor OS rate (P = 0.031). Serum P1NP levels at or above 75 ng/mL correlate with a short time to development of bone metastasis and low overall survival in patients with stage I–III breast cancer.

Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Scheid V, Buzdar AU, Smith TL, Hortobagyi GN (1986) Clinical course of breast cancer patients with osseous metastasis treated with combination chemotherapy. Cancer 58:2589–2593
Alexandraki I (2011) The United States-Mexico border: an area in need of cancer screening interventions. J Women’s Health 20:653–655
Mehta RS, Barlow WE, Albain KS et al (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367:435–444
Lipton A (2003) Bone metastases in breast cancer. Curr Treat Options Oncol 4:151–158
Lipton A, Costa L, Ali S, Demers L (2001) Use of markers of bone turnover for monitoring bone metastases and the response to therapy. Semin Oncol 28:54–59
Bellahcene A, Kroll M, Liebens F, Castronovo V (1996) Bone sialoprotein expression in primary human breast cancer is associated with bone metastases development. J Bone Miner Res 11:665–670
Buijs JT, Henriquez NV, van Overveld PG et al (2007) Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res 67:8742–8751
Chao TY, Yu JC, Ku CH et al (2005) Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res 11:544–550
Diel IJ, Solomayer EF, Seibel MJ et al (1999) Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin Cancer Res 5:3914–3919
Wada N, Ishii S, Ikeda T, Enomoto K, Kitajima M (1999) Serum tartrate resistant acid phosphatase as a potential marker of bone metastasis from breast cancer. Anticancer Res 19:4515–4521
Wu YY, Janckila AJ, Ku CH et al (2010) Serum tartrate-resistant acid phosphatase 5b activity as a prognostic marker of survival in breast cancer with bone metastasis. BMC Cancer 10:158
Lipton A, Costa L, Ali SM, Demers LM (2001) Bone markers in the management of metastatic bone disease. Cancer Treat Rev 27:181–185
Samoszuk M, Leuther M, Hoyle N (2008) Role of serum P1NP measurement for monitoring treatment response in osteoporosis. Biomark Med 2:495–508
Lipton A (2008) Emerging role of bisphosphonates in the clinic–antitumor activity and prevention of metastasis to bone. Cancer Treat Rev 34(Suppl 1):S25–S30
Nangia JR, Ma JD, Nguyen CM, Mendes MA, Trivedi MV (2012) Denosumab for treatment of breast cancer bone metastases and beyond. Expert Opin Biol Ther 12:491–501
Barton MK (2011) Denosumab an option for patients with bone metastasis from breast cancer. CA Cancer J Clin 61:135–136
Henry DH, Costa L, Goldwasser F et al (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29:1125–1132
Lipton A, Steger GG, Figueroa J et al (2007) Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol 25:4431–4437
Andersen PK, Gill RD (1982) Cox’s regression model for counting processes: a large sample study. Ann Stat 10:1100–1120
Tawara K, Oxford JT, Jorcyk CL (2011) Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res 3:177–189
Koizumi M, Yonese J, Fukui I, Ogata E (2001) The serum level of the amino-terminal propeptide of type I procollagen is a sensitive marker for prostate cancer metastasis to bone. BJU Int 87:348–351
Thurairaja R, Iles RK, Jefferson K, McFarlane JP, Persad RA (2006) Serum amino-terminal propeptide of type 1 procollagen (P1NP) in prostate cancer: a potential predictor of bone metastases and prognosticator for disease progression and survival. Urol Int 76:67–71
Oremek G, Sauer-Eppel H, Klepzig M (2007) Total procollagen type 1 amino-terminal propeptide (total P1NP) as a bone metastasis marker in gynecological carcinomas. Anticancer Res 27:1961–1962
Pollmann D, Siepmann S, Geppert R, Wernecke KD, Possinger K, Luftner D (2007) The amino-terminal propeptide (PINP) of type I collagen is a clinically valid indicator of bone turnover and extent of metastatic spread in osseous metastatic breast cancer. Anticancer Res 27:1853–1862
Marin L, Koivula MK, Jukkola-Vuorinen A, Leino A, Risteli J (2011) Comparison of total and intact aminoterminal propeptide of type I procollagen assays in patients with breast cancer with or without bone metastases. Ann Clin Biochem 48:447–451
Saarto T, Blomqvist C, Risteli J, Risteli L, Sarna S, Elomaa I (1998) Aminoterminal propeptide of type I procollagen (PINP) correlates to bone loss and predicts the efficacy of antiresorptive therapy in pre- and post-menopausal non-metastatic breast cancer patients. Br J Cancer 78:240–245
Tahtela R, Tholix E (1996) Serum concentrations of type I collagen carboxyterminal telopeptide (ICTP) and type I procollagen carboxy-and aminoterminal propeptides (PICP, PINP) as markers of metastatic bone disease in breast cancer. Anticancer Res 16:2289–2293
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529
McCloskey E, Paterson A, Kanis J, Tahtela R, Powles T (2010) Effect of oral clodronate on bone mass, bone turnover and subsequent metastases in women with primary breast cancer. Eur J Cancer 46:558–565
Esteva FJ, Guo H, Zhang S et al (2010) PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol 177:1647–1656
Ali SM, Esteva FJ, Hortobagyi G et al (2001) Safety and efficacy of bisphosphonates beyond 24 months in cancer patients. J Clin Oncol 19:3434–3437
Esteva FJ, Valero V, Pusztai L, Boehnke-Michaud L, Buzdar AU, Hortobagyi GN (2001) Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist 6:133–146
Esteva FJ (2004) Monoclonal antibodies, small molecules, and vaccines in the treatment of breast cancer. Oncologist 9:4–9
Aapro MS (2011) Denosumab for bone metastases from breast cancer: A new therapy option? J Clin Oncol 29: e419–e420; author reply e421–e414
de Kluyver RL, Sayers TJ (2010) Breast cancer bone metastases: combination therapy targeting cancer cells and the tumor microenvironment. Cancer Biol Ther 9:551–553
Fizazi K, Lipton A, Mariette X et al (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27:1564–1571
Lee BL, Higgins MJ, Goss PE (2012) Denosumab and the current status of bone-modifying drugs in breast cancer. Acta Oncol 51:157–167
van der Pluijm G (2011) Breast cancer bone metastases: denosumab or zoledronic acid? Nat Rev Endocrinol 7:134–135
Paterson AH, Anderson SJ, Lembersky BC et al (2012) Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol 13:734–742
Body JJ, Dumon JC, Gineyts E, Delmas PD (1997) Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer 75:408–412
Fornander T, Rutqvist LE, Wilking N, Carlstrom K, von Schoultz B (1993) Oestrogenic effects of adjuvant tamoxifen in postmenopausal breast cancer. Eur J Cancer 29A:497–500
Kong QQ, Sun TW, Dou QY et al (2007) Beta-CTX and ICTP act as indicators of skeletal metastasis status in male patients with non-small cell lung cancer. Int J Biol Markers 22:214–220
Leeming DJ, Delling G, Koizumi M et al (2006) Alpha CTX as a biomarker of skeletal invasion of breast cancer: immunolocalization and the load dependency of urinary excretion. Cancer Epidemiol Biomark Prev 15:1392–1395
Brasso K, Christensen IJ, Johansen JS et al (2006) Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate 66:503–513
Kim SW, Kim JS, Papadopoulos J et al (2011) Consistent interactions between tumor cell IL-6 and macrophage TNF-alpha enhance the growth of human prostate cancer cells in the bone of nude mouse. Int Immunopharmacol 11:862–872
Tang CH, Chuang JY, Fong YC, Maa MC, Way TD, Hung CH (2008) Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-kappaB pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis 29:1483–1492
Tsangari H, Findlay DM, Kuliwaba JS, Atkins GJ, Fazzalari NL (2004) Increased expression of IL-6 and RANK mRNA in human trabecular bone from fragility fracture of the femoral neck. Bone 35:334–342
Blair JM, Zhou H, Seibel MJ, Dunstan CR (2006) Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol 3:41–49
Wall KM, Nunez-Rocha GM, Salinas-Martinez AM, Sanchez-Pena SR (2008) Determinants of the use of breast cancer screening among women workers in urban Mexico. Prev Chronic Dis 5:A50
Acknowledgments
We thank our patients for providing the blood samples for our biomarker research. This study was supported by an investigator-initiated grant provided by Roche Diagnostics to Francisco J. Esteva. This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Conflict of interest
The authors made no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dean-Colomb, W., Hess, K.R., Young, E. et al. Elevated serum P1NP predicts development of bone metastasis and survival in early-stage breast cancer. Breast Cancer Res Treat 137, 631–636 (2013). https://doi.org/10.1007/s10549-012-2374-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2374-0