Abstract
Introduction
Procollagen type I N-terminal propeptide (P1NP), a bone formation marker, reportedly predicts bone mineral density (BMD) response to teriparatide treatment in treatment-naive patients with osteoporosis. Results from a randomized, phase 3, open-label, active-controlled trial— STRUCTURE—showed that in patients previously treated with bisphosphonates, romosozumab led to gains in hip BMD, which were not observed with teriparatide. This post hoc analysis investigated the comparative utility of early changes in P1NP in predicting BMD response in patients who participated in the STRUCTURE trial, which enrolled patients who switched treatment from bisphosphonates to romosozumab/teriparatide.
Materials and methods
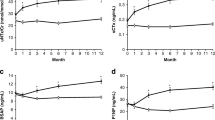
Postmenopausal women (aged 55–90 years) with osteoporosis who had previously taken bisphosphonates were randomized to receive open-label subcutaneous romosozumab (210 mg once monthly; n = 218) or teriparatide (20 µg once daily; n = 218) for 12 months. BMD was assessed by dual-energy X-ray absorptiometry at the proximal femur and lumbar spine (LS) at baseline and months 6 and 12. To assess the utility of P1NP, the positive predictive value of increase from baseline in P1NP of > 10 µg/L at month 1 and achievement of various thresholds of percent change from baseline in BMD at month 12 were evaluated.
Results
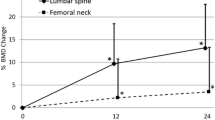
Overall, 95% (191/202) of patients in the romosozumab group and 91% (183/201) in the teriparatide group demonstrated an increase in P1NP of > 10 µg/L from baseline at month 1. Among these patients, 18% and 3% of romosozumab-treated patients versus 60% and 12% of teriparatide-treated patients showed no increase from baseline (i.e., ≤ 0%) in total hip and LS BMD, respectively, at month 12. These data indicate that in patients switching from bisphosphonates to a bone-forming therapy, increases in P1NP do not help predict the hip BMD response. Although most patients treated with either teriparatide or romosozumab showed an increase in P1NP, the majority of patients on romosozumab showed an increase in hip BMD, while more than half of the patients on teriparatide did not. Teriparatide therapy did not increase total hip BMD in the majority of patients who transitioned from bisphosphonates to teriparatide.
Conclusions
Thus, increases in P1NP were not predictive of BMD response in the teriparatide group because in approximately 60% of the patients who were administered teriparatide, the hip BMD decreased independent of the change in P1NP levels.


Similar content being viewed by others
Change history
20 March 2020
In the original publication of the article, the last row of Table��1 was published incorrectly as ���Serum P1NP (��mol/L), median (IQR)b : Romosozumab, 25 (18, 34); Teriparatide, 25 (20, 33)���. The correct row should be read as ���Serum P1NP (��g/L), median (IQR)b : Romosozumab, 25 (18, 34); Teriparatide, 25 (20, 33)���.
Notes
BMD bone mineral density, BTM bone turnover marker, CTX C-telopeptide of type 1 collagen, DXA dual-energy X-ray absorptiometry, FN femoral neck, LS lumbar spine, P1NP procollagen type I N-terminal propeptide, QD once a day, QM once a month, SC subcutaneous, TH total hip.
References
Imaz I, Zegarra P, González-Enríquez J, Rubio B, Alcazar R, Amate JM (2010) Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int 21:1943–1951. https://doi.org/10.1007/s00198-009-1134-4
Okazaki R, Muraoka R, Maehara M, Inoue D (2019) Factors associated with inadequate responses to risedronate in Japanese patients with osteoporosis. J Bone Miner Metab 37:185–197. https://doi.org/10.1007/s00774-018-0931-2
Krege JH, Lane NE, Harris JM, Miller PD (2014) PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int 25:2159–2171. https://doi.org/10.1007/s00198-014-2646-0
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89:1117–1123. https://doi.org/10.1210/jc.2003-030501
Delmas PD, Vrijens B, Roux C et al (2003) A reinforcement message based on bone turnover marker response influences long-term persistence with risedronate in osteoporosis: the IMPACT study. J Bone Miner Res 18:S374. https://doi.org/10.1002/jbmr.5650181306
Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH (2011) PINP as an aid for monitoring patients treated with teriparatide. Bone 48:798–803. https://doi.org/10.1016/j.bone.2010.12.006
Nishizawa Y, Ohta H, Miura M, Inaba M, Ichimura S, Shiraki M, Takada J, Chaki O, Hagino H, Fujiwara S, Fukunaga M, Miki T, Yoshimura N (2013) Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition). J Bone Miner Metab 31:1–15. https://doi.org/10.1007/s00774-012-0392-y
Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93:3785–3793. https://doi.org/10.1210/jc.2008-0353
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T (2008) Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23:1591–1600. https://doi.org/10.1359/jbmr.080506
Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP et al (2017) Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 390:1585–1594. https://doi.org/10.1016/s0140-6736(17)31613-6
Eastell R, Krege JH, Chen P, Glass EV, Reginster J-Y (2006) Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr Med Res Opin 22:61–66. https://doi.org/10.1185/030079905x75096
Gallagher JC, Rosen CJ, Chen P, Misurski DA, Marcus R (2006) Response rate of bone mineral density to teriparatide in postmenopausal women with osteoporosis. Bone 39:1268–1275. https://doi.org/10.1016/j.bone.2006.06.007
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CAF, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543. https://doi.org/10.1056/NEJMoa1607948
Ishibashi H, Crittenden DB, Miyauchi A, Libanati C, Maddox J, Fan M, Chen L, Grauer A (2017) Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone 103:209–215. https://doi.org/10.1016/j.bone.2017.07.005
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster J-Y, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang Y-C, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420. https://doi.org/10.1056/NEJMoa1305224
Ma YL, Zeng QQ, Chiang AY, Burr D, Li J, Dobnig H, Fahrleitner-Pammer A, Michalská D, Marin F, Pavo I, Stepang JJ (2014) Effects of teriparatide on cortical histomorphometric variables in postmenopausal women with or without prior alendronate treatment. Bone 59:139–147. https://doi.org/10.1016/j.bone.2013.11.011
Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK (2011) Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int 22:357–362. https://doi.org/10.1007/s00198-010-1226-1
Moreira CA, Fitzpatrick LA, Wang Y, Recker RR (2017) Effects of abaloparatide-SC (BA058) on bone histology and histomorphometry: the ACTIVE phase 3 trial. Bone 97:314–319. https://doi.org/10.1016/j.bone.2016.11.004
Tsai JN, Uihlein AV, Burnett-Bowie SAM, Neer RM, Zhu Y, Derrico N, Lee H, Bouxsein ML, Leder BZ (2015) Comparative effects of teriparatide, denosumab, and combination therapy on peripheral compartmental bone density, microarchitecture, and estimated strength: the DATA-HRpQCT study. J Bone Miner Res 30:39–45. https://doi.org/10.1002/jbmr.2315
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. Medical writing support was provided by Avinash Thakur and Frances Gambling of Cactus Communications and was funded by AABP. Qualified researchers may request data from Amgen clinical studies. Complete details are available at: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.
Funding
Amgen Inc., and UCB Pharma sponsored this study. All costs associated with the development of this manuscript, including medical writing, were funded by Amgen Inc., Astellas, and UCB Pharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rajani Dinavahi, Cassandra E. Milmont, and Andreas Grauer are employees of Amgen Inc., USA, and report holding stocks of Amgen Inc. Etsuro Hamaya, Toshiyasu Hirama, and Yoichi Nakamura are employees of Amgen Astellas BioPharma K.K., Japan. Additionally, Etsuro Hamaya and Toshiyasu Hirama report holding stocks of Amgen Inc. Cesar Libanati is an employee of UCB Pharma, Belgium, and reports holding stocks of UCB Pharma. Junichi Takada and Akimitsu Miyauchi have nothing to disclose.
Ethical approval
This study was done in accordance with International Conference on Harmonization Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. An independent ethics committee or institutional review board at each site approved the protocol, informed consent form, and all protocol amendments.
Informed consent
Patients gave written informed consent before any study-specific procedures were done.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Takada, J., Dinavahi, R., Miyauchi, A. et al. Relationship between P1NP, a biochemical marker of bone turnover, and bone mineral density in patients transitioned from alendronate to romosozumab or teriparatide: a post hoc analysis of the STRUCTURE trial. J Bone Miner Metab 38, 310–315 (2020). https://doi.org/10.1007/s00774-019-01057-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01057-1